Abstract
Introduction
In children with hypertrophic cardiomyopathy (HCM) there often occurs a non-ischemic pattern of myocardial fibrosis, which could be the cause of impaired left ventricular (LV) diastolic function assessed by tissue Doppler imaging (TDI). The aim of the study was to determine the prevalence of myocardial fibrosis in children with HCM, and to evaluate its relationship with echocardiographic parameters including LV diastolic dysfunction.
Material and methods
Sixty-three children with HCM, mean age 12.2 ±4.5 years, underwent magnetic resonance imaging (MRI) and echocardiographic study from January 2010 to April 2014. The results of MRI, echocardiography, and TDI velocities were analyzed and compared between children with and without myocardial fibrosis. Moreover, correlations between the results of echocardiography and MRI were assessed.
Results
Our results showed a significant correlation between magnetic resonance and echocardiographic measurements of septal wall thickness, posterior wall thickness, LV mass and left atrial dimension. Children with myocardial fibrosis (60%) had a significantly thicker interventricular septum (21.3 vs. 1.8 mm; p < 0.0001) and larger left atrial dimension (36.7 vs. 27.8 mm; p = 0.0004) and volume index (42.0 vs. 26.6 ml/m²; p = 0.0011). Tissue Doppler imaging demonstrated significantly decreased lateral E’ (9.02 vs. 13.53 cm/s; p < 0.0001) and septal E′ (7.05 vs. 9.36 cm/s; p = 0.0082) velocities and a significantly increased transmitral lateral (10.34 vs. 6.68; p = 0.0091) and septal (13.1 vs. 9.8; p = 0.046) E/E′ ratio in children with myocardial fibrosis.
Conclusions
Myocardial fibrosis in children with hypertrophic cardiomyopathy was associated with markers for disease severity such as larger septum thickness, enlargement of the left atrium as well as impairment of left ventricular diastolic function. Tissue Doppler imaging is a helpful tool to detect the presence of left ventricular diastolic dysfunction in children with hypertrophic cardiomyopathy and myocardial fibrosis.
Keywords: hypertrophic cardiomyopathy, magnetic resonance imaging, myocardial fibrosis, left ventricular diastolic dysfunction
Introduction
Hypertrophic cardiomyopathy (HCM) is a relatively common genetic cardiac disease, accounting for 42% of childhood cardiomyopathy [1]. The severity of cardiac hypertrophy, etiology, as well as the clinical course of HCM in children is varied, resulting in a large spectrum of clinical and phenotypic expression [2, 3]. Many patients develop symptoms of breathlessness due to diastolic dysfunction which is largely independent of the severity of left ventricular (LV) outflow tract obstruction [4]. As patients with HCM frequently demonstrate a non-ischemic pattern of myocardial fibrosis, this may represent a mechanism for increased myocardial stiffness leading to impaired diastolic filling [5]. Magnetic resonance imaging (MRI) with late gadolinium enhancement (LGE) can detect a small and focal myocardial fibrosis [6]. Magnetic resonance imaging has been widely accepted for detection of myocardial fibrosis, which has been implicated as a factor in cardiovascular events and LV function [7]. Recently, echocardiographic modalities such as tissue Doppler imaging (TDI) have become a sensitive measure of diastolic ventricular dysfunction. They are relatively load independent and can reliably determine the degree of LV diastolic dysfunction, which affects the clinical course in children with HCM [8].
The aim of this study was to determine the prevalence of myocardial fibrosis assessed by MRI in children with HCM and to evaluate its relationship with echocardiographic parameters including LV diastolic dysfunction.
Material and methods
Study patients
Sixty-three children with HCM hospitalized in the Department of Pediatric Cardiology underwent MRI between January 2010 and April 2014. Criteria for inclusion in the study were age < 18 years at the time of diagnosis and echocardiographic evidence of myocardial hypertrophy defined as a diastolic septal thickness or LV diastolic wall thickness z-score > 2 (determined as more than two standard deviations from the mean value for the population indexed to the body surface area), in the absence of hemodynamic conditions that could account for the observed hypertrophy. The study was reviewed and approved by the Institutional Ethics Committee. Individual informed consent was obtained.
Data collection
Patients’ demographics, clinical symptoms (dyspnea on exertion, syncope, chest pain), arrhythmias, heart failure, family history of HCM and sudden cardiac death (SCD) as well as the results of echocardiography, MRI, resting 12-lead and 24-h Holter electrocardiography were collected.
Family history of SCD was defined as one or more cardiac death in first-degree relatives of < 40 years of age with or without a diagnosis of HCM or SCD in first-degree relatives with confirmed hypertrophy at any age [9]. Unexplained syncope was defined as unexplained transient loss of consciousness at or prior to first evaluation. Two-dimensional, conventional pulsed Doppler, TDI and M-mode echocardiography were performed at rest using standard methods (ultrasound machine iE 33, Philips, Healthcare). Echocardiographic measurements included septal wall thickness (SWT) and LV posterior wall thickness (LVPWT) (mm, z-score), left atrial (LA) size (mm, z-score), LA volume indexed to the body surface area (BSA) and presence of LV outflow tract obstruction (LVOTO). LVOTO was considered present when a peak instantaneous outflow gradient of ≥ 30 mm Hg was calculated with continuous-wave Doppler echocardiography at rest. Left atrial dimension was measured at end-systole as the antero-posterior linear diameter from the parasternal long-axis view. Left atrial enlargement was defined as a z-score > 2. Left atrial volume and LV mass were determined by the truncated ellipsoid method and M-mode, respectively, for each patient and were indexed to BSA. The SWT, LVPWT in diastole, and LA dimension were evaluated for each patient and compared with the reference values indexed for BSA as stated in the literature [10] using the calculator available online [Kapmann et al. Heart 2000 parameterz.com]. Z-scores for the SWT, LVPWT and the LA dimension were calculated using the formula for z-scores as reported in the literature [11]. Hypertrophic cardiomyopathy was diagnosed when the diastolic septal thickness or left ventricular diastolic wall thickness gave a z-score > 2 (determined as more than two standard deviations from the mean value for the population indexed to the body surface area), in the absence of hemodynamic conditions that could account for the observed LV hypertrophy [9]. Left atrial volume was considered increased when the value was > 28 ml/m². Impaired LV diastolic function was defined as mild when the LA volume was 29–33 ml/m², moderate when it was 34–39 ml/m², and severe when it was > 40 ml/m² [12]. Conventional pulsed Doppler was used to record the mitral inflow pattern at the leaflet tips in the apical 4-chamber view. Peak velocities of E and A waves (cm/s) and their ratio (E/A) were measured. Velocity of E and A waves as well as E/A ratio were evaluated for each patient and related to the reported normal values [13]. Z-scores of the above parameters were calculated using previously reported formulas [11]. The TDI was obtained from an apical 4-chamber view to receive longitudinal annular velocities at the lateral mitral wall and septum. Early diastolic (E′) and late diastolic (A′) tissue Doppler velocities (cm/s) were measured at the mitral lateral and septal walls and subsequently averaged over 3 cardiac cycles. Transmitral E/E′ ratios (lateral and septal) were calculated for each patient. Velocities of E′ and A′ and the E/E′ ratio were evaluated for each patient and compared with previously reported normal values [13]. Z-scores of E′ and A′ velocities and the E/E′ ratio were calculated using the calculator available online [Eidem et al. JASE 2004 parameterz.com]. The values of E and A waves, E/A ratio and tissue Doppler parameters were considered abnormal if they were decreased or elevated as compared to normal values for a certain age. Twenty-four-hour Holter monitors were reviewed to determine the incidence of significant arrhythmias. Nonsustained ventricular tachycardia was defined as ≥ 3 consecutive ventricular extrasystoles at a rate of ≥ 120 beats/min lasting < 30 s during Holter monitoring [9]. Electrocardiograms were reviewed and all abnormalities were documented.
Magnetic resonance imaging was performed on a 1.5 T scanner (Siemens, Germany). Cine images were acquired with steady-state free-precession technique (trueFISP; slice thickness 8 mm) in three long-axis planes and contiguous short-axis slices (trueFISP; slice thickness 8 mm, 2 mm gap) from the atrioventricular ring to the apex. An intravenous bolus of 0.1 mmol/kg of gadobutrol (Gadovist, Schering, Berlin, Germany) or gadodiamide (Omniscan, GE Healthcare, United Kingdom in patients below 2 years of age) was then given and late gadolinium images were acquired in the same planes after 10 min, with a breath-hold segmented inversion-recovery sequence (inversion time: 280–400 ms). Global LV function was analyzed using commercially available software by manual tracing of endocardial and epicardial contours (Argus, Siemens Medical Solutions, Germany). The following parameters (indexed to BSA) were calculated: LV end-diastolic volume index (ml/m2), LV end-systolic volume index (ml/m2), LV ejection fraction (%), LV mass index (g/m2). End-diastolic septal and LVPWT was measured in the short axis (mm). Left atrial dimension was measured in the LV outflow tract view in systole and its enlargement was defined as a z-score > 2. The presence of left ventricular LGE was determined using visual assessment by two independent observers and quantified as mild (discrete limited LGE seen in up to two successive short axis slices), moderate (well-defined LGE seen in at least two successive short axis slices) or severe (extensive, well-defined LGE in multiple short axis slices).
Methods of analysis
All children were divided into a group with myocardial fibrosis by MRI and a group without fibrosis. The results of echocardiography and tissue Doppler studies were analyzed and compared between the two groups.
Statistical analysis
Statistical analysis was performed using MedCalc statistical software (version 12.4, MedCalc Software, Ostend, Belgium). Results are presented as mean values ± standard deviation. Nominal variables are expressed as the number of subjects and the percentage in the analyzed group. Comparisons of echocardiographic parameters between patients according to presence or absence of fibrosis as detected by late gadolinium enhancement MRI were made with the unpaired t test or χ2 test for continuous and categorical data respectively. Pearson’s correlation coefficients were calculated for selected paired echocardiographic and corresponding MRI parameters.
Results
Clinical and demographic data
Sixty-three children with HCM were included in the study. The mean age at diagnosis of the disease was 6.2 years (range from 0.01 to 17 years), and the mean age at MRI and tissue Doppler study was 12.2 years (range from 1.4 to 17.9 years). Fifteen (23.8%) patients had a family history of SCD and 29 (46%) subjects had a family history of HCM. Thirty-six (57%) children were in NYHA class II, and 7 (11%) were in NYHA class III/IV. Altogether, 37.9% of patients presented with clinical symptoms such as syncope (7.9%) or chest pain (30%). In 4.8% of children nonsustained ventricular tachycardia on 24-h Holter electrocardiography was observed. Fifteen (23.8%) patients had LV outflow tract obstruction with a peak systolic pressure gradient of above 30 mm Hg. Sixty (95%) children were treated with β-blockers and 3 (4.8%) with calcium channel blockers. Baseline characteristics of the study group are presented in Table I.
Table I.
Patient characteristics
| Clinical parameters | Total N = 63 |
|---|---|
| Age at diagnosis [years] | 6.2 ±5.7 |
| Age at baseline MRI, echo [years] | 12.2 ±4.5 |
| Male (%) | 42 (67) |
| BSA at baseline MRI [m2] | 1.4 ±0.4 |
| NYHA functional class, n (%): | |
| I | 20 (32) |
| II | 36 (57) |
| III/IV | 7 (11) |
| Mean NYHA functional class | 1.8 ±0.6 |
| Family history of HCM, n (%) | 29 (46) |
| Family history of SCD, n (%) | 15 (23.8) |
| Syncope, n (%) | 5 (7.9) |
| Chest pain, n (%) | 19 (30) |
| Peak wall thickness on echo [mm] | 17.6 ±8.5 |
| Peak wall thickness on echo, z-score | 10.1±7.2 |
| LV mass/BSA on echo [g/m2] | 149.3 ±97.3 |
| LA dimension [mm] | 33.2 ±10.1 |
| LA dimension, z-score | 1.7 ±2.7 |
| Wall thickness > 30 mm, n (%) | 8 (12.7) |
| Rest LVOTO > 30 mm Hg, n (%) | 15 (23.8) |
| Nonsustained VT, n (%) | 3 (4.8) |
| QTc dispersion | 0.048 ±0.015 |
| β-blockers, n (%) | 60 (95) |
| Calcium antagonist, n (%) | 3 (4.8) |
| MRI findings: | |
| LVEF (%) | 66.5 ±7.4 |
| LVEDV/BSA [ml/m2] | 82.1 ±17.9 |
| LVESV/BSA [ml/m2] | 27.4 ±9.9 |
| LV mass/BSA [g/m2] | 100.1 ±60.7 |
| Peak wall thickness on MRI [mm] | 20.3 ±8.8 |
| Peak wall thickness on MRI, z-score | 13.4 ±8.2 |
| LA dimension [mm] | 29.3 ±9.6 |
| LA dimension, z-score | 0.6 ±2.7 |
Values are mean ± SD unless otherwise stated. MRI – magnetic resonance imaging, BSA – body surface area, HCM – hypertrophic cardiomyopathy, SCD – sudden cardiac death, LV – left ventricular, LVEDV – left ventricular end-diastolic volume, LVEF – left ventricular ejection fraction, LVESV – left ventricular end-systolic volume, LVOTO – left ventricular outflow tract obstruction, VT – ventricular tachycardia, LA – left atrium, NYHA – New York Heart Association functional class.
Magnetic resonance imaging findings and their correlation with echocardiographic parameters
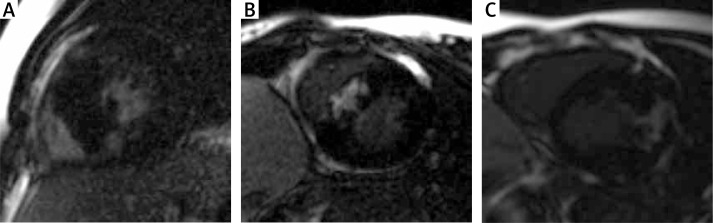
Magnetic resonance imaging was successfully completed in all 63 patients. The results are summarized in Table I. In the MRI study maximum SWT was higher than in echocardiography (p = 0.02, mean z-score 13.4 vs. 10.1, respectively), while LV mass indexed to BSA and LA dimension were smaller in comparison to results of echocardiography (p = 0.0006, 100 vs. 149 g/m² and the mean z-score 0.6 vs. 1.7, respectively). Myocardial fibrosis (MF) was observed in 38 (60%) children. Among them 27 patients had mild myocardial fibrosis, 7 patients had moderate MF and 4 patients were found to have severe MF. In the majority of studies MF was observed in the insertion points (17) and/or ventricular septum (17). In the remaining subjects (7) MF was found in the lateral wall or papillary muscles. Subendocardially-based myocardial fibrosis, consistent with ischemic scarring, was not observed (Figures 1 A–C).
Figure 1.
Distribution of late gadolinium enhancement in the study group: A – Moderate extent of insertion point fibrosis, B – severe extent of intraventricular septum fibrosis, C – severe myocardial fibrosis of hypertrophied antero- lateral LV wall
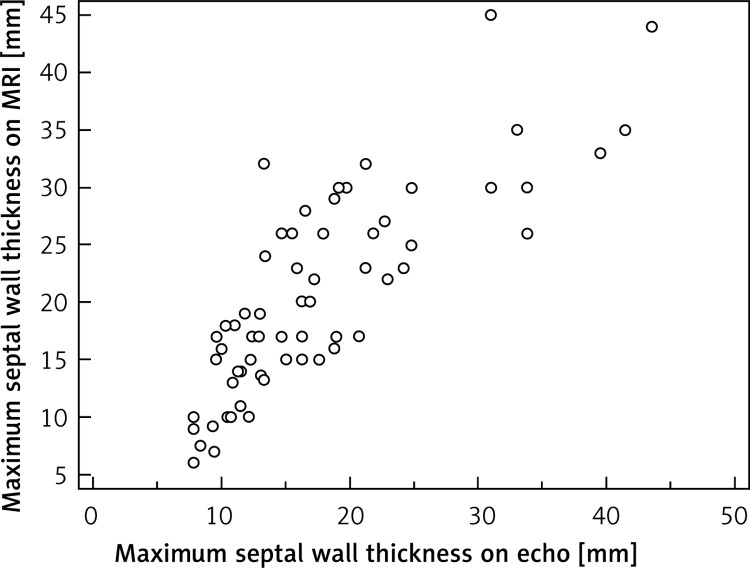
We found a very good correlation between MRI and echo measurements of the maximum septal wall thickness (r = 0.81, p < 0.0001), as shown in Figure 2.
Figure 2.
Correlation of echocardiographic parameters with MRI in 63 children with hypertrophic cardiomyopathy. Maximum septal wall thickness (r = 0.81, p < 0.0001)
There was also a significant positive correlation between the above two modalities in the assessment of the maximum LVPWT, LV mass and LA diameter (r = 0.64, p < 0.0001; r = 0.66, p < 0.0001; r = 0.72, p ≤ 0.0001, respectively).
Echocardiographic and tissue Doppler findings in patients with and without myocardial fibrosis
Based on MRI findings, children were classified as either patients with myocardial fibrosis according to MRI (n = 38; 60%) or without fibrosis (n = 25; 40%). The comparison of the echocardiographic variables, including tissue Doppler velocities, between patients with and without myocardial fibrosis is presented in Table II.
Table II.
Differences in echocardiographic parameters between patients with and without fibrosis on MRI
| Parameters | Patients with MF (n = 38) | Patients without MF (n = 25) | P-value |
|---|---|---|---|
| SWT [mm] | 21.3 ±8.7 | 11.8 ±3.7 | < 0.0001 |
| SWT (z-score) | 12.9 ±7.1 | 5.8 ±5 | 0.00006 |
| LVPWT [mm] | 10.3 ±4.1 | 7.7 ±2.4 | 0.0065 |
| LVPWT (z-score) | 2.2 ±2.6 | 1.3 ±3.3 | 0.24 |
| Rest LVOTO > 30 mm Hg, n (%) | 13 (34.2) | 2 (8) | 0.0369 |
| LA dimension [mm] | 36.7 ±11.2 | 27.8 ±4.6 | 0.0004 |
| LA dimension (z-score) | 2.5 ±3.1 | 0.5 ±1.1 | 0.003 |
| LA volume indexed to BSA [ml/m2] | 42.0 ±21.6 | 26.6 ±7.2 | 0.0011 |
| LV diastolic dysfunction, n (pts) | Mild: 6 Moderate: 3 Severe: 18 Norm: 11 |
Mild: 5 Moderate: 0 Severe: 2 Norm: 18 |
0.0036 |
| LV diastolic dysfunction, n (%) | 27 (71) | 7 (28) | 0.0004 |
| Mitral inflow: | |||
| E velocity [cm/s] | 88.1 ±35.2 | 86.3 ±14.4 | 0.81 |
| E velocity, z-score | –0.2 ±2 | –0.4 ±0.9 | 0.65 |
| A velocity [cm/s] | 56.3 ±18.7 | 55.9 ±13.1 | 0.93 |
| A velocity, z-score | 0.6 ±1.4 | 0.5 ±0.9 | 0.86 |
| E/A ratio | 1.8 ±1.23 | 1.62 ±0.47 | 0.49 |
| E/A, z-score | –0.3 ±1.86 | –0.7 ±0.82 | 0.43 |
| TDI mitral annular velocities [cm/s]: | |||
| Lateral E′ | 9.02 ±3.6 | 13.53 ±3.0 | < 0.0001 |
| Lateral E′, z-score | –2.78 ±1.1 | –1.41 ±0.9 | < 0.0001 |
| Lateral A′ | 6.93 ±2.9 | 8.15 ±2.3 | 0.12 |
| Lateral A′, z-score | 0.15 ±1.7 | 0.85 ±1.3 | 0.14 |
| Transmitral lateral E/E′ | 10.34 ±5.3 | 6.68 ±3.2 | 0.0091 |
| Transmitral lateral E/E′, z-score | 3.78 ±4.1 | 0.82 ±1.5 | 0.0033 |
| Septal S′ | 6.78 ±1.8 | 7.40 ±1.4 | 0.1967 |
| Septal S′, z-score | –1.22 ±1.3 | –0.64 ±0.9 | 0.07 |
| Septal E′ | 7.05 ±3.1 | 9.36 ±2.7 | 0.0082 |
| Septal E′, z-score | –3.1 ±1.4 | –2.06 ±1.1 | 0.0069 |
| Septal A′ | 5.86 ±1.3 | 6.55 ±1.2 | 0.0628 |
| Septal A′, z-score | –0.21 ±0.9 | 0.35 ±0.8 | 0.027 |
| Transmitral septal E/E′ | 13.1 ±6.6 | 9.83 ±4.1 | 0.0461 |
| Transmitral septal E/E′, z-score | 4.2 ±4.5 | 1.82 ±2.3 | 0.027 |
Values are mean ± SD unless otherwise stated. MF – myocardial fibrosis, SWT – septal wall thickness in diastole, LVPWT – left ventricular posterior wall thickness in diastole, LV – left ventricular, LA – left atrium, LVOTO – left ventricular outflow tract obstruction, BSA – body surface area, TDI – tissue Doppler imaging.
Children with myocardial fibrosis had a significantly thicker interventricular septum (p < 0.0001), larger LA dimension (p = 0.003) and higher LA volume index (p = 0.001) compared to patients without fibrosis. Left ventricular diastolic dysfunction determined by the increased volume of the LA was observed significantly more frequently in children with myocardial fibrosis (p = 0.0004). Moderate LV diastolic dysfunction (LA volume index between 34 and 39 ml/m²) or severe diastolic dysfunction (LA volume index > 40 ml/m²) was diagnosed in 33% of patients with and in 3% of children without fibrosis (p = 0.0036). Of the 63 analyzed patients, in 5 moderate mitral insufficiency was present, and in the remaining children mitral insufficiency was a trace, hemodynamically insignificant or absent. All patients with moderate mitral insufficiency were in the group with MF. If we excluded them from the analysis, LA volume indexed to the BSA was 37.7 ±18 ml/m² in the MF group and 26.6 ±7.3 ml/m² in the group without MF (the difference was still statistically significant, p = 0.0054).
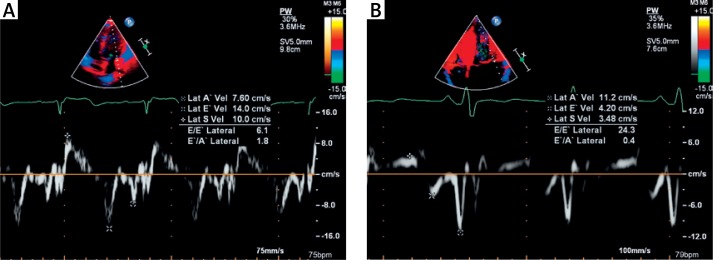
LVOTO with gradient > 30 mm Hg at rest occurred significantly more often in patients with myocardial fibrosis (p = 0.0036). There were no significant differences in mitral inflow Doppler velocities between the groups. The tissue Doppler profile demonstrated significantly decreased early diastolic lateral E′ and septal E′ mitral annular velocities in children with fibrosis (p < 0.0001 and p = 0.0069, respectively) compared to patients without it, as shown in Figures 3 A, B.
Figure 3.
A – Tissue Doppler imaging demonstrating lateral mitral annulus velocity in patient with hypertrophic cardiomyopathy without myocardial fibrosis and normal diastolic function. B – Tissue Doppler imaging demonstrating lateral mitral annulus velocity in patient with hypertrophic cardiomyopathy, with myocardial fibrosis and diastolic dysfunction. Note that the patient with diastolic dysfunction had a decreased early diastolic annular velocity E′ but an increased late diastolic velocity A′, resulting in E′/A′ reversal and an increased E/E′ ratio, which is typical for left ventricular diastolic dysfunction
Tissue Doppler velocity at the late diastolic lateral A′ was lower in children with myocardial fibrosis, although this difference was not significant (p = 0.14). However, septal A′ velocity was significantly decreased in patients with fibrosis (p = 0.027). Transmitral lateral and septal E/E′ ratios were significantly increased in children with myocardial fibrosis (p < 0.0001 and p = 0.027, respectively).
Discussion
Correlation of echocardiographic and MRI parameters
Hypertrophic cardiomyopathy is characterized by LV hypertrophy with a subsequent increase in LV mass and in some patients with LA enlargement. Echocardiography remains an essential first-line test in these patients. The purpose of our study was to compare the diagnostic ability of echocardiography with MRI and to assess the additive clinical value of MRI in children with HCM. Our results showed a significant positive correlation between MRI and echocardiography measurements of the maximum SWT, maximum LVPWT, LV mass and LA dimension. However, differences between MRI and echo measurements of peak wall thickness and LA dimensions were noted. In our study MRI provided better spatial resolution and 3D as opposed to 2D in the echo approach to LV assessment. Therefore MRI should be considered as a reference method while echo seems to possibly underestimate LV wall thickness and overestimate LA dimension in HCM.
Moreover, our findings emphasize that MRI can provide additional information of which myocardial fibrosis is the most relevant.
Influence of myocardial fibrosis on left ventricular diastolic function in children with hypertrophic cardiomyopathy
Myocardial hypertrophy is often accompanied by myocardial fibrosis. Histology revealed increased amounts of fibrous tissue not solely in the interventricular septum, but also in the LV free wall and even in the right ventricle [14]. It is considered that myocardial fibrosis is the consequence of microvascular ischemia, which triggers apoptosis and collagen deposition [15]. Our study demonstrated that children with HCM had a high prevalence of myocardial fibrosis (60%). Recent studies using new imaging modalities such as MRI with LGE indicated that myocardial fibrosis was associated with LV dysfunction [16, 17]. In adult patients with HCM, the presence of fibrosis according to MRI correlated with LV wall thickness and LA size [18–20]. Similarly, the results of our study showed that children with myocardial fibrosis had significantly increased septum thickness, a higher LVPWT, and significantly larger LA dimension and volume index on echocardiography compared to patients without fibrosis. Prinz et al. [21] reported that LV and LA size correlated with the presence and severity of fibrosis on MRI in adult patients with HCM. It should be particularly noted that in our study impaired LV diastolic function was found significantly more often in children with myocardial fibrosis. As many as 47% of children with fibrosis had severe LV diastolic dysfunction as compared to 8% of patients without fibrosis. On the other hand, almost 30% of subjects with myocardial fibrosis had normal diastolic function defined as indexed left atrial area. It seems that further prospective studies in the field are necessary, as possibly diastolic dysfunction develops later with the progression of myocardial fibrosis. Furthermore, it should be emphasized that with the available data it appears that the correlation between diastolic function and hypertrophy and LGE is relevant and significant at the population level, but it may fail in a single patient.
In our study group obstructive HCM and systolic pressure gradient > 30 mm Hg were significantly more frequent in children with fibrosis. To our knowledge, the occurrence and role of myocardial fibrosis have been assessed in only a few studies in children [22] and in adults with HCM [23, 24].
Usefulness of echocardiography with tissue Doppler imaging in the evaluation of left ventricular diastolic dysfunction in children with hypertrophic cardiomyopathy and myocardial fibrosis
Conventional echocardiographic Doppler indices that evaluate diastolic ventricular function are suboptimal, mostly because of their dependence on loading conditions [25]. Among adults with HCM, transmitral Doppler inflow velocities have been shown to correlate poorly with symptoms, exercise capacity, mean LA pressure, and LV hypertrophy [25–27]. In the study of McMahon et al. [26] it was found that in children with HCM, transmitral E- and A-wave velocities failed to distinguish between patients who reached the clinical end points. Significant variation in mitral inflow velocities with altered loading conditions makes this an unreliable echocardiographic predictor of diastolic dysfunction in children with HCM [26]. Similarly, the results of our study indicated that transmitral E- and A-wave velocities did not differentiate patients with or without myocardial fibrosis and LV diastolic dysfunction.
Tissue Doppler imaging should be used with standard echocardiographic measurements to estimate LV filling pressure in patients with HCM. Previous results, using early mitral inflow to early diastolic mitral annular velocity ratio (E/E′) as a non-invasive measure of increased LV filling pressure and thus LV diastolic dysfunction, suggested a significant link between high LV filling pressures and myocardial fibrosis in patients with HCM [27–29]. Our results are comparable and showed that MRI-based presence of myocardial fibrosis was strongly correlated with tissue Doppler velocity indices. To our knowledge this is the first study to demonstrate that children with myocardial fibrosis in MRI have significantly lower lateral and septal early diastolic tissue velocities (E′) as well as a significantly higher lateral and septal E/E′ ratio compared to patients without fibrosis. Ellims et al. [30] reported similar findings in an adult population with HCM. In adult studies, increased LV end-diastolic pressure is consistent with an increased E/E′ ratio, and this may play a significant role in the development of symptoms, worsening NYHA class, and impairment of LV diastolic function [31–34]. McMahon et al. [26] demonstrated that high septal E/E′ ratio predicted major clinical events and risk of death in children with HCM. The results of our research, like the study of Avegliano et al. [35], showed that the presence of dynamic LVOTO affected the degree of LV diastolic dysfunction (mitral annular E′ velocity was significantly lower and the E/E′ ratio was significantly higher in patients with LV obstruction compared to children without obstruction). Other authors also emphasize the important pathophysiological role of LVOTO in patients with HCM, and they suggest performing obstruction-provoking tests such as sublingual spray application of isosorbide dinitrate and the Valsalva maneuver for evaluation of LVOT obstructions, which are clinically relevant and can be managed using specific surgical and non-surgical interventions [36].
As in the study by Wu et al. [37], the results of our research showed that LA enlargement was significantly associated with tissue Doppler-based diastolic dysfunction and abnormal tissue Doppler imaging velocities.
Clinically, the results of our study demonstrated that children with HCM and myocardial fibrosis had increased prevalence of tissue Doppler-based LV diastolic dysfunction. Determining the presence of myocardial fibrosis in MRI may be helpful in the identification of children with diastolic dysfunction when correlated with tissue Doppler imaging variables.
There were some limitations in our study design. The presence of myocardial fibrosis in the left ventricle was visually analyzed, along with late gadolinium enhancement distribution pattern and location. The study lacked quantitative assessment of myocardial fibrosis identified by late gadolinium enhancement, which will be examined in further studies.
In conclusion, myocardial fibrosis in children with hypertrophic cardiomyopathy was associated with markers for disease severity such as greater septum thickness, enlargement of the left atrium as well as impairment of left ventricular diastolic function. Echocardiography with tissue Doppler imaging is a feasible and sensitive method for evaluating left ventricular diastolic dysfunction in children with hypertrophic cardiomyopathy and myocardial fibrosis. The transmitral lateral and septal E/E′ ratio allows early detection of left ventricular diastolic dysfunction in children with hypertrophic cardiomyopathy.
Acknowledgments
This work was supported by the National Science Centre, ul. Królewska 57, 30-081 Krakow, Poland (Grant No. 1481/B/P01/2011/40).
The authors gratefully acknowledge the support of Jolanta Misko MD PhD, Lukasz Malek MD PhD, Lukasz Mazurkiewicz MD PhD, and Mateusz Spiewak MD PhD from the Cardiac Magnetic Resonance Unit, Institute of Cardiology, Warsaw and the support of colleagues from the Laboratory of Echocardiography at the Children’s Memorial Health Institute. Without their assistance, this paper would not have been possible.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Lipshultz SE, Sleeper LA, Towbin JA, et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N Eng J Med. 2003;348:1647–55. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ. Hypertrophic cardiomyopathy in childhood. Paediatr Clin North Am. 2004;51:1305–34. doi: 10.1016/j.pcl.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Nugent A, Daubeney P, Chondros P, et al. Clinical features and outcomes of childhood hypertrophic cardiomyopathy: results from a national population-based study. Circulation. 2005;112:1332–8. doi: 10.1161/CIRCULATIONAHA.104.530303. [DOI] [PubMed] [Google Scholar]
- 4.Fifer MA, Vlahakes GJ. Management of symptoms in hypertrophic cardiomyopathy. Circulation. 2008;117:429–39. doi: 10.1161/CIRCULATIONAHA.107.694158. [DOI] [PubMed] [Google Scholar]
- 5.Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000;84:476–82. doi: 10.1136/heart.84.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–74. doi: 10.1016/j.jacc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Bachinski LL, Meyer D, et al. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation. 2001;104:128–30. doi: 10.1161/01.cir.104.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–79. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 10.Kampmann C, Wiethoff CM, Wenzel A, et al. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart. 2000;83:667–72. doi: 10.1136/heart.83.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chubb H, Simpson JM. The use Z-score in paediatric cardiology. Ann Pediatr Cardiol. 2012;5:179–84. doi: 10.4103/0974-2069.99622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Eidem BW, McMahon CJ, Cohen RR, et al. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004;17:212–21. doi: 10.1016/j.echo.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Unverferth DV, Baker PB, Pearce LI, Lautman J, Roberts WC. Reginal myocyte hypertrophy and increased interstitial myocardial fibrosis in hypertrophic cardiomyopathy. Am J Cardiol. 1987;59:932–36. doi: 10.1016/0002-9149(87)91128-3. [DOI] [PubMed] [Google Scholar]
- 15.Girolami F, Ho CY, Semsatian C, et al. Clinical features and outcome of hypertrophic cardiomyopathy associated with triple sarcomere protein gene mutations. J Am Coll Cardiol. 2010;55:1444–53. doi: 10.1016/j.jacc.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 16.Rubinshtein R, Glockner JF, Ommen SR, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Cir Heart Fail. 2010;3:51–8. doi: 10.1161/CIRCHEARTFAILURE.109.854026. [DOI] [PubMed] [Google Scholar]
- 17.Rochitte CE, Tassi EM, Shiozaki AA. The emerging role of MRI in the diagnosis and management of cardiomyopathies. Curr Cardiol Rep. 2006;8:44–52. doi: 10.1007/s11886-006-0010-5. [DOI] [PubMed] [Google Scholar]
- 18.Choudhury L, Mahrholdt H, Wagner A, et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156–64. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 19.Maron MS, Appelbaum E, Harrigan CJ, et al. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail. 2008;1:184–91. doi: 10.1161/CIRCHEARTFAILURE.108.768119. [DOI] [PubMed] [Google Scholar]
- 20.Prinz C, van Buuren F, Faber L, et al. Myocardial fibrosis is associated with biventricular dysfunction in patients with hypertrophic cardiomyopathy. Echocardiography. 2012;29:438–44. doi: 10.1111/j.1540-8175.2011.01588.x. [DOI] [PubMed] [Google Scholar]
- 21.Prinz C, Hering D, Bitter T, Horstkotte D, Faber L. Left atrial size and left ventricular hypertrophy correlate with myocardial fibrosis in patients with hypertrophic cardiomyopathy. Acta Cardiol. 2011;66:153–7. doi: 10.1080/ac.66.2.2071245. [DOI] [PubMed] [Google Scholar]
- 22.Hussain T, Dragulescu A, Benson L, et al. Diffuse myocardial fibrosis in pediatric hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2013;15(Suppl. 1):072. [Google Scholar]
- 23.Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012;5:370–7. doi: 10.1016/j.jcmg.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Ellims AH, Iles LM, Ling L, et al. A comprehensive evaluation of myocardial fibrosis in hypertrophic cardiomyopathy with cardiac magnetic resonance imaging: linking genotype with fibrotic phenotype. Eur Heart J Cardiovasc Imaging. 2014;15:1108–16. doi: 10.1093/ehjci/jeu077. [DOI] [PubMed] [Google Scholar]
- 25.Maron BJ, Spirito P, Green KJ, et al. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1987;10:733–42. doi: 10.1016/s0735-1097(87)80264-4. [DOI] [PubMed] [Google Scholar]
- 26.McMahon CJ, Nagueh SF, Pignatelli RH, et al. Characterization of left ventricular diastolic function by tissue Doppler imaging and clinical status in children with hypertrophic cardiomyopathy. Circulation. 2004;109:1756–62. doi: 10.1161/01.CIR.0000124723.16433.31. [DOI] [PubMed] [Google Scholar]
- 27.Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH, Zoghbi WA, Quinones MA. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99:254–61. doi: 10.1161/01.cir.99.2.254. [DOI] [PubMed] [Google Scholar]
- 28.Matsumara Y, Elliott PM, Virdee MS, Sorajja P, Doi Y, McKenna WJ. Left ventricular diastolic function assessed using Doppler tissue imaging in patients with hypertrophic cardiomyopathy: relation to symptoms and exercise capacity. Heart. 2002;87:247–51. doi: 10.1136/heart.87.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiography. 2009;10:165–93. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 30.Ellims AH, Iles LM, Ling LH, Hare JL, Kaye DM, Taylor AJ. Diffuse myocardial fibrosis in hypertrophic cardiomyopathy can be identified by cardiovascular magnetic resonance, and is associated with left ventricular diastolic dysfunction. J Cardiovasc Magn Reson. 2012;14:64–76. doi: 10.1186/1532-429X-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efthimiadis GK, Giannakoulas G, Parcharidou DG, et al. Clinical significance of tissue Doppler imaging in patients with hypertrophic cardiomyopathy. Circ J. 2007;71:897–903. doi: 10.1253/circj.71.897. [DOI] [PubMed] [Google Scholar]
- 32.Bayrak F, Kahveci G, Mutlu B, Sonmez K, Degertekin M. Tissue Doppler imaging to predict clinical course of patients with hypertrophic cardiomyopathy. Eur J Echocardiography. 2008;9:278–83. doi: 10.1093/ejechocard/jen049. [DOI] [PubMed] [Google Scholar]
- 33.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura Y, Elliott PM, Virdee MS, et al. Left ventricular diastolic function assessed using Doppler tissue imaging in patients with hypertrophic cardiomyopathy: relation to symptoms and exercise capacity. Heart. 2002;87:247–51. doi: 10.1136/heart.87.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avegliano G, Costabel JP, Huguet M, et al. Influence of dynamic obstruction and hypertrophy location on diastolic function in hypertrophic cardiomyopathy. J Cardiovasc Med. 2014;15:207–13. doi: 10.2459/JCM.0b013e3283638093. [DOI] [PubMed] [Google Scholar]
- 36.Zemanek D, Tomasov P, Bělehrad M, et al. Comparison of sublingual isosorbide dinitrate and Valsalva maneuver for detection of obstruction in hypertrophic cardiomyopathy. Arch Med Sci. 2015;11:1–5. doi: 10.5114/aoms.2015.47096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu V, Chyou JY, Chung S, Bhagavatula S, Axel L. Evaluation of diastolic function by three-dimensional volume tracking of the mitral annulus with cardiovascular magnetic resonance: comparison with tissue Doppler imaging. J Cardiovasc Magnet Reson. 2014;16:71–84. doi: 10.1186/s12968-014-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]