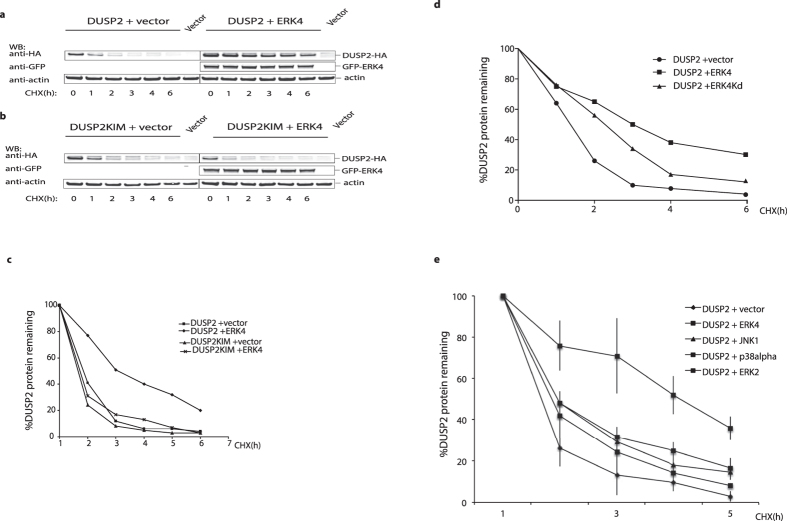
Figure 7. ERK4 stabilizes the DUSP2 protein in vivo.
(a,b) HeLa cells were co-transfected with expression vectors encoding either DUSP2-HA (a) or DUSP2KIM-HA (b) together with either an empty expression vector or a plasmid encoding GFP-ERK4. After 24 h, cells were treated with 10 μM cycloheximide (CHX) for the indicated time periods before cell harvesting and lysis. The levels of DUSP2-HA or DUSP2KIM-HA in whole cell extracts were analysed by Western blotting using an anti-HA antibody. Levels of GFP-ERK4 and endogenous actin in the cell extracts were analysed by Western blotting using antibodies against GFP and actin, respectively. Unprocessed original scans of the blots are shown in Supplementary Fig. 1 (c) The intensities of the signals corresponding to the DUSP2 and DUSP2KIM bands in both a and b, were then quantified using the Odyssey infrared imaging System. The band intensities at the indicated time points are then expressed graphically as a percentage of the intensity at time zero. (d) A kinase-dead mutant of ERK4 stabilizes DUSP2 less efficiently than wild-type ERK4. Experiments were performed as described in (a–c). (e) Co-expression of classical MAP kinases does not lead to stabilization of the DUSP2 protein. HeLa cells were co-transfected with an expression vector encoding HA-tagged wild type DUSP2 together with expression vectors encoding either myc- tagged ERK4, ERK2, p38α or JNK1. Cycloheximide time course experiments, followed by Western blot analyses and quantification were performed exactly as described in a-c. The average of three independent experiments is shown including standard deviation. The experiments (a–d) were performed three times with identical results and the results of a single representative experiment are shown.