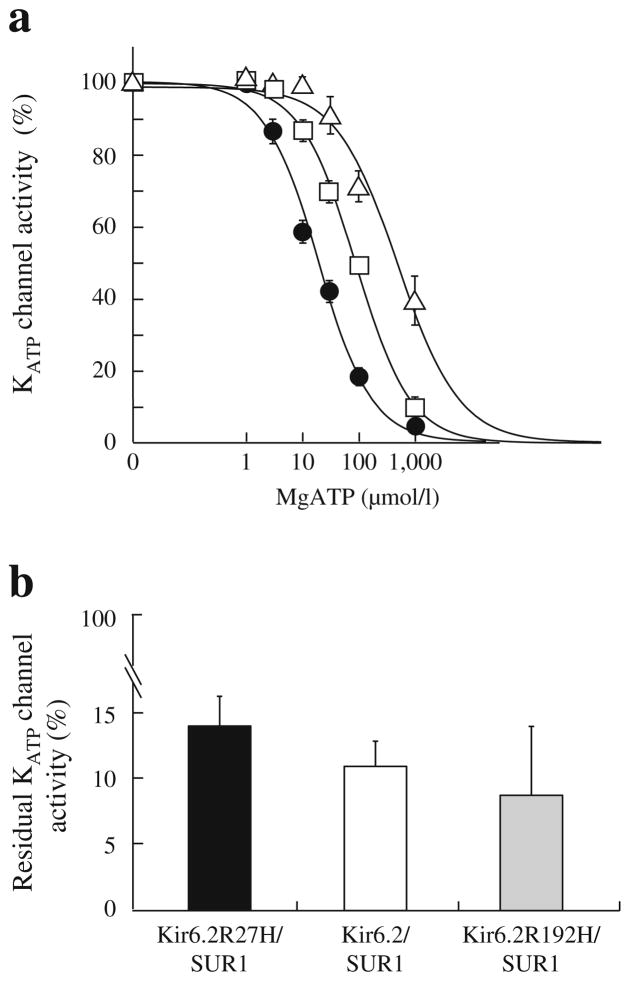
Fig. 3.
ATP sensitivity and sulfonylurea sensitivity of the wild-type and mutant KATP channels. (a) ATP sensitivity. Patch-clamp experiments were performed for the wild-type (Kir6.2/SUR1, black circles, n =10) and mutant (Kir6.2 R27H/SUR1, white squares, n =10; Kir6.2 R192H/ SUR1, white triangles, n =8) channels. The mutant channels had significantly higher IC50 values than the wild-type channels (92.3±9.6 vs 20.2±2.4 μmol/l, p= 2.66×10−5; 652.7±178.0 vs 20.2±2.4 μmol/l, p= 9.14×10−7), indicating that the mutant KATP channels had lower ATP sensitivities, especially the Kir6.2 R192H/SUR1 channel. (b) Sulfonylurea sensitivity. Residual KATP channel activities were determined by the ratio between the amplitudes of KATP channel currents before and after 100 μmol/l tolbutamide application. Experiments were conducted for wild-type (white column, n =15) and mutant (black column, Kir6.2 R27H/SUR1, n =10; Kir6.2 R192H/SUR1, grey column, n =9) channels. No significant difference in sulfonylurea sensitivity was observed for the mutant channels compared with the wild-type channels (p >0.05)