Abstract
Purpose:
To demonstrate that vasoactive intestinal peptide (VIP), a corneal endothelial (CE) cell autocrine factor, maintains the integrity of corneal endothelium in human donor corneoscleral explants precut for endothelial keratoplasty.
Methods:
Twelve paired human donor corneoscleral explants used as control versus VIP-treated explants (10 nM, 30 minutes, 37°C) were shipped (4°C) to the Lions Eye Institute for Transplantation and Research for precutting (Moria CBM-ALTK Keratome), shipped back to the laboratory, and cultured in ciliary neurotrophic factor (CNTF, 0.83 nM, 37°C, 24 hours). Trephined endothelial discs (8–8.5 mm) were analyzed for differentiation markers (N-cadherin, CNTF receptor α subunit [CNTFRα], and connexin 43) by Western blot after a quarter of the discs from 4 paired explants were cut away and stained with alizarin red S for microscopic damage analysis. Two additional paired explants (6 days in culture) were stained for panoramic view of central CE damage.
Results:
VIP treatment increased N-cadherin and CNTFRα levels (mean ± SEM) to 1.38 ± 0.11-fold (P = 0.003) and 1.46 ± 0.22-fold (P = 0.03) of paired controls, respectively, whereas CE cell CNTF responsiveness in upregulation of connexin 43 increased to 2.02 ± 0.5 (mean ± SEM)-fold of the controls (P = 0.04). CE damage decreased from (mean ± SEM) 10.0% ± 1.2% to 1.6% ± 0.3% (P < 0.0001) and 9.1% ± 1.1% to 2.4% ± 1.0% (P = 0.0006). After 6 days in culture, the damage in whole CE discs decreased from 20.0% (control) to 5.5% (VIP treated).
Conclusions:
VIP treatment before precut enhanced the preservation of corneal endothelium.
Key Words: human corneal endothelial cell, differentiation, survival, Descemet stripping automated endothelial keratoplasty
Among organ transplantations, human corneal transplantation is unique in that the graft is avascular1 and not subjected to ischemia/reperfusion injury. Nevertheless, the human donor cornea is subjected to significant mechanical force during graft preparation and grafting itself. When examined 6 months after transplantation,2–5 the graft typically shows 30% corneal endothelial (CE) cell loss, which has long been considered a factor contributing to graft failure.6,7 The causes remain unspecified, with only a correlation between the incision width and CE cell loss.8
The corneal endothelium is without a surplus of CE (stem) cells, and maintenance of its integrity through corneoscleral explant dissection, preservation, and keratoplasty is of critical importance. Our long-term goal is to identify factors that maintain the differentiated state of corneal endothelium. We have shown that, through the concerted actions of its autocrine trophic factors, vasoactive intestinal peptide (VIP) and ciliary neurotrophic factor (CNTF), the corneal endothelium plays an active role in maintaining its own differentiated state as a monolayer of hexagonal cells and in promoting self-survival in stored human donor corneoscleral explants.9
Endogenous VIP maintains in the corneal endothelium the level of the adhesion molecule N-cadherin,10 whereas exogenous VIP upregulates CE N-cadherin in human donor corneoscleral explants.10 Knocking down endogenous VIP gene diminishes N-cadherin, dramatically deteriorates the CE mosaic in which hexagonal cells are replaced by irregularly shaped, larger ones, and lowers CE cell density.10 N-cadherin is a regulator11,12 and a marker of CE differentiation.13 N-cadherin is critical for the structure and function of the corneal endothelium. N-cadherin-knockout mice demonstrate apical junctional complex disorganization, tight junction continuity interruption, CE permeability increase, and cell shape alteration.14
VIP gene and protein expression in the corneal endothelium of human donor corneoscleral explants are upregulated by CNTF,15 an injury factor discovered in an extract of the ciliary body, iris, and choroid.16 CNTF does not have the signal sequence for secretion,18 but is an autocrine factor of the corneal endothelium. CNTF is released in conjunction with the CNTF-binding CNTF receptor α subunit (CNTFRα) by the corneal endothelium surviving oxidative stress.17 CE CNTFRα19 is gradually lost from corneoscleral explants in storage (4°C).19 The recombinant CNTFRα places itself in the CE cell membrane and restores functional CNTFRα.19 A brief VIP treatment of human donor corneoscleral explants before or during their storage upholds the CE CNTFRα level and increases the responsiveness to exogenous CNTF stimulation upregulating the gap junctional protein connexin 43.20 CNTF upregulates connexin 43,20,21 whereas connexin 43-mediated gap junctional communication is essential for resisting oxidative stress,22 which in turn decreases connexin 43 expression.23 Connexin 43 hemichannels are passages for released ATP-driving intercellular communication.24 A brief treatment of human donor corneoscleral explants with VIP (37°C) before or during their storage in storage medium (4°C) increases long-term CE cell retention and reduces damage to the corneal endothelium.20
CE cells express the VIP receptor VPAC1 (but not VPAC2).20,25 VIP protects the corneal endothelium against the killing effect of acute oxidative stress ex vivo.26 VIP stimulates glycogen breakdown and upholds the ATP level in the corneal endothelium under oxidative stress, allowing the switch of the death mode from inflammatory necrosis to inflammatory neutral apoptosis while upregulating the antiapoptotic Bcl-2 and N-cadherin in a kinase A-dependent manner.25
According to the Eye Bank Association of America (2014),27 endothelial keratoplasty has become the most commonly performed corneal transplantation procedure in the United States. It is therefore critical that the beneficial effects of VIP treatment remain in precut corneas.
MATERIALS AND METHODS
Scheme 1
Paired human donor corneoscleral explants treated as control versus VIP-treated explants were precut, organ cultured in CNTF, and analyzed for endothelial integrity.
SCHEME 1.
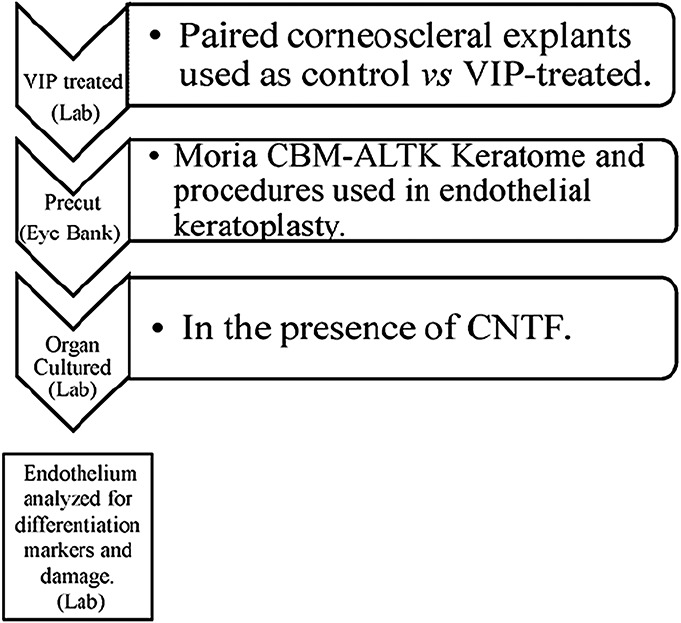
Paired human donor corneoscleral explants treated as control vs VIP-treated prior to precut were precut, organ cultured in CNTF, and analyzed for endothelial integrity.
Media
1) Medium A: Eagle minimal essential medium with Earl salts plus 20 mM HEPES and 2 mM glutamine; 2) medium B: medium A supplemented with penicillin (200 U/mL) and streptomycin sulfate (200 μg/mL); 3) storage medium: Optisol-GS (Bausch & Lomb, Rochester, NY).
Human Donor Corneoscleral Explants
Fresh Human Donor Corneoscleral Explants
Following the same procedures as the eye bank, fresh human donor corneoscleral explants were retrieved from deceased donors (within 30 hours postmortem) in the Maryland State Anatomy Board and placed in Dulbecco's phosphate-buffered saline (DPBS) on ice. Serology was performed through Tissue Banks International (Baltimore, MD). Deceased donors were deidentified and not considered as human subjects by the Human Research Protection Office of the university.
Preserved Human Donor Corneoscleral Explants
Viable human corneoscleral explants in storage medium (4°C) were from the Lions Eye Institute for Transplant and Research, Inc (Tampa, FL).
VIP Treatment of Human Donor Corneoscleral Explants
Fresh Human Donor Corneoscleral Explants
Before their storage (4°C), human donor corneoscleral explants dissected from the right eyes were treated with VIP (V6130; Sigma, St Louis, MO; 10−8 M in medium A), whereas those of the paired left eyes were used as controls and treated with medium A alone in 35-mm Petri dishes containing 3.5 mL of medium A under 5% CO2/95% air at 37°C for 30 minutes. The corneoscleral explants were stored (4°C) for 4 days before being shipped to the Lions Eye Institute for cornea precutting.
Preserved Human Donor Corneoscleral Explants
Explants previously in storage were treated using the same method as that used for the fresh human donor corneoscleral explants described above (except that explants from the left eyes were VIP treated and those from the right eyes were controls).
Precutting of Corneas of Corneoscleral Explants
One to 2 days after being shipped from the laboratory, corneas were precut at the Lions Eye Institute, using a Moria CBM-ALTK keratome (Moria, Doylestown, PA) and procedures identical to those in the preparation of corneal tissues to be used for lamellar or endothelial keratoplasty.28 After precut, human donor corneoscleral explants were placed in fresh storage medium and shipped back to the laboratory (4°C).
Organ Culture of Precut Corneoscleral Explants
After their arrival at the laboratory, the paired human donor corneoscleral (control and VIP-treated) explants were incubated in medium A for 1 hour, followed by incubation in medium B supplemented with CNTF (0.83 nM) for either 24 hours or 6 days at 5% CO2/95% air and 37°C.
Cell Extract From Trephined Discs
Discs of corneal endothelium were trephined (8 or 8.5 mm) from the center of the precut corneas. The cell extract of the CE discs was obtained from either the whole disc (4 pairs) or 75% of the discs (2 pairs) homogenized in the RIPA buffer as described.20
Western Blot Analysis
Cell extracts were electrophoresed [reducing conditions, preformed Tris/glycine polyacryl amide gradient gels (Nu-Page; Novex, San Diego, CA)] and electrophoretically transferred to nitrocellulose membranes and were analyzed using the following primary antibodies: affinity-purified polyclonal antibodies against N-cadherin [BTA7 (R&D Systems), affinity-purified rabbit-anti-connexin 43 (AB19012; Chemicon), affinity-purified goat anti-human CNTFRα (AF-303-NA; R&D Systems), and mouse monoclonal anti-actin (CP01, anti-actin [Ab-1; Calbiochem]). Densities of markers were quantified and normalized against actin. Both control [Con] and VIP-treated [VIP] explants were divided by controls and ratios were expressed in the y-axis.
Alizarin Red S Staining of the Damaged Corneal Endothelium
After removal of the undissolved dye by centrifugation, the saturated alizarin red S solution (prepared from 1% alizarin red S [Sigma, Fluka 5600] in calcium- and magnesium-free DPBS [Gibco; 14190]) was used without pH adjustment. After rinsing explants twice with Ca+2-Mg2+-free DPBS corneal cups were covered with the saturated alizarin red S solution for 180 seconds. Explants were finally rinsed in DPBS 2 or 3 times and photographed for quantification of the red stain.
Photography
Digital photomicrographs (3 × 10−3 mm2/field) of the CE sheet were taken under an inverted light microscope (Diaphot; Nikon, Tokyo, Japan). Panoramic view of the alizarin red S-stained corneal endothelium was captured after placement of the explants on a light box (CE side up).
Quantification of Damaged Areas in the Corneal Endothelium
Alizarin red S-stained areas of damaged corneal endothelium revealed in the photographs were quantified using Adobe Photoshop CS6 computer software following closely the method described by Saad et al.29 The damage was expressed as a percentage by dividing the number of pixels in the damaged area by the number of pixels in the entire image.
RESULTS
N-Cadherin Level in the Corneal Endothelium of Precut Corneas Increased by Prior VIP Treatment of Human Donor Corneoscleral Explants
VIP was effective in increasing the N-cadherin level in the corneal endothelium of 5 of the 6 human donor corneoscleral pairs. The only pair (donor 5, Table 1) that did not show the effect of VIP was from a donor with subarachnoid hemorrhage indicated as the cause of death and with blood cells adhering to the corneal endothelium (data not shown). In all 6 pairs, CE N-cadherin levels in VIP-treated corneoscleral explants were 1.0- to 1.71-fold of their respective controls (1.38 ± 0.11 [mean ± SEM]; P = 0.003; Fig. 1; Table 1). There was no correlation between VIP responsiveness and the donor's age (Table 1).
TABLE 1.
Paired Human Corneoscleral Explants From 6 Donors: Effects of VIP Treatment on CE N-Cadherin and Connexin 43 in Precut Corneas
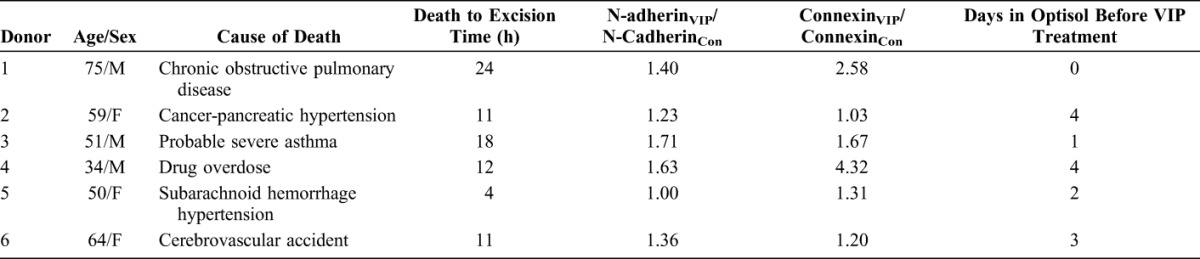
FIGURE 1.
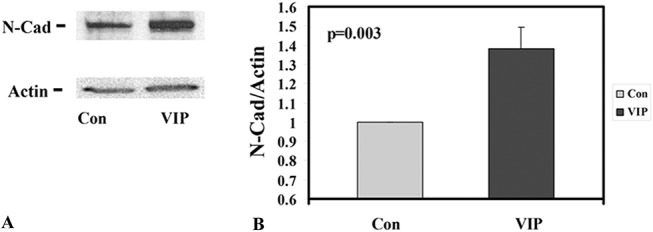
VIP treatment before cornea precut increased CE N-cadherin levels. A, Western blot analysis for N-cadherin (N-Cad) and actin (internal standard) of control (Con) and VIP-treated (VIP) explants (pair 2, Table 1). B, Normalized N-cadherin levels (against actin) in all 6 pairs of explants showing 1.38 ± 0.11 (mean ± SEM)-fold of increase from the untreated paired controls (P = 0.003).
Enhanced CNTF Responsiveness (in Upregulation of the Connexin 43 Level) in CE Cells of Precut Corneas Resulting From Previous VIP Treatment of Human Donor Corneoscleral Explants
VIP treatment (see Materials and Methods) before cornea precut increased the CE responsiveness to CNTF modulation (in upregulation of connexin 43) in cultured human donor corneoscleral explants. In all 6 pairs, VIP treatment demonstrated increased connexin 43 levels that were 1.03- to 4.32-fold of their respective controls (2.02 ± 0.5 [mean ± SEM]; P = 0.04; Fig. 2, Table 1).
FIGURE 2.
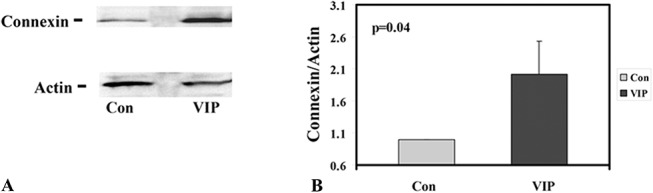
VIP treatment before cornea precut increased CNTF responsiveness of the corneal endothelium (in upregulating connexin 43). A, Western blot analysis for CE connexin 43 and actin (as internal standard) of control (Con) and VIP-treated (VIP) paired corneoscleral explants (pair 2, Table 1). B, Normalized connexin 43 (against actin) of all 6 pairs of explants increased to 2.02 ± 0.5 (mean ± SEM)-fold of the control (P = 0.04).
VIP Treatment of Human Donor Corneoscleral Explants Prevented Loss of CNTFRα From the Corneal Endothelium of Precut Corneas
CE cells in human donor corneoscleral explants in storage (4°C) gradually lose their CNTFRα and that functional CNTFRα can be restored by treatment of the explants with recombinant CNTFRα.19 A brief VIP treatment of fresh corneoscleral explants before their storage (for 4, 5, 10, and 25 days) results in increases in retention of CE CNTFRα, relative to those found in untreated paired corneoscleral explants.20 From fresh donor corneas, only one species of CE CNTFRα (53 kDa) was detected.15 In the present study, organ cultured in the presence of the ligand CNTF, corneal endothelium expressed an additional 47-kDa CNTFRα, whose level was increased by the VIP pretreatment (Fig. 3). The relationship between the 53-kDa and 47-kDa CNTFRα molecules is unknown. Recombinant CNTFRα has been shown to form a 1:1 complex with CNTF, and the complex assumes a smaller molecular size than the recombinant CNTFRα.30 Relative 47-kDa CNTFRα levels found in the control and VIP-treated paired explants from 6 pairs were 1.00 and 1.46 ± 0.22 (mean ± SEM; P = 0.03; Fig. 3), respectively.
FIGURE 3.
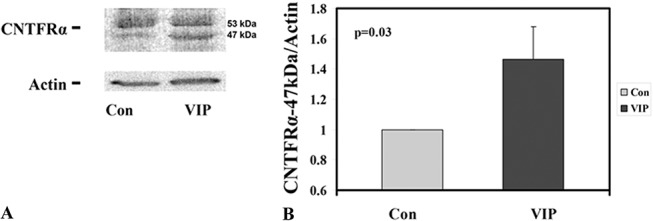
VIP treatment before cornea precut enhanced CE CNTFRα preservation. A, Western blot analysis for CNTFRα and actin (internal standard) from pair 2 (Table 1) demonstrating the increased 47-kDa CNTFRα level in the VIP-treated corneoscleral explant. B, Relative 47-kDa CNTFRα levels found in the control and VIP-treated paired corneoscleral explants from all 6 pairs were 1.00 and 1.46 ± 0.22 (mean ± SEM; P = 0.03), respectively.
Cultured Precut Human Donor Corneoscleral Explants With Brief VIP Treatment Before Cornea Precut Demonstrated Decreased CE Damage
A quarter of CE discs trephined from each of the 2 pairs (1 and 3) of corneoscleral explants was cut away and stained with alizarin red S for microscopic viewing of CE damage. In pair 1 (Fig. 4), the control and VIP-treated corneoscleral explants demonstrated CE damage of 9.98% ± 1.24% and 1.61% ± 0.31%, respectively (mean ± SEM; P < 0.0001; n = 53 images). In pair 3 (Fig. 5), the damaged areas in the control and VIP-treated corneoscleral explants were 9.05% ± 1.12% and 2.40% ± 0.94%, respectively (mean ± SEM; P < 0.0001; n = 27 images). Thus, a brief (30 minutes) VIP (10−8 M) treatment of corneoscleral explants before their precut reduced levels of CE damage to 16% of the control level in pair 1 and to 27% of the control level in pair 3. To corroborate the results shown in Figures 4 and 5, panoramic views of the whole corneal discs from a pair of 6-day cultured corneoscleral explants were analyzed for CE damage (Fig. 6) in a preliminary study. The damaged areas in the control and VIP-treated explants were 20% and 5.5%, respectively (Fig. 6).
FIGURE 4.
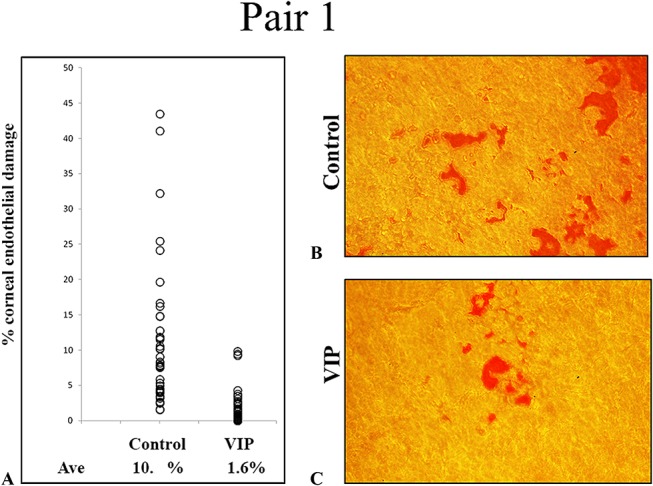
Reduced CE damage by VIP treatment before cornea precut demonstrated microscopically (pair 1, Table 1). A, Control and VIP-treated explants demonstrated damaged areas of (mean ± SEM) 9.98% ± 1.24% and 1.61% ± 0.31%, respectively (53 images, P < 0.0001). B and C, Alizarin red S staining of the exposed Descemet membrane in denuded (damaged) corneal endothelium of control (B) and VIP-treated (C) explants. Original magnification ×200. Ave, average.
FIGURE 5.
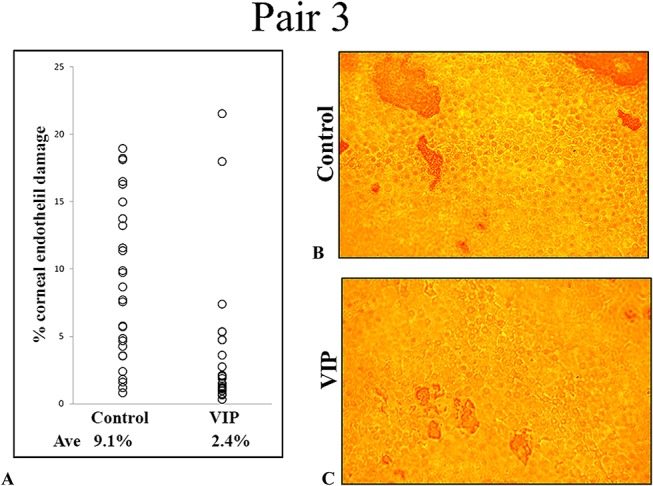
Reduced CE damage by VIP treatment before cornea precut demonstrated microscopically (pair 3, Table 1). A, Control and VIP-treated explants demonstrated damaged areas of (mean ± SEM) 9.05% ± 1.12% and 2.40% ± 0.94%, respectively (27 images, P = 0.0006). B and C, Alizarin red S staining of the exposed (damaged) Descemet membrane in corneal endothelium of control (B) and VIP-treated (C) explants. Original magnification ×200. Ave, average.
FIGURE 6.
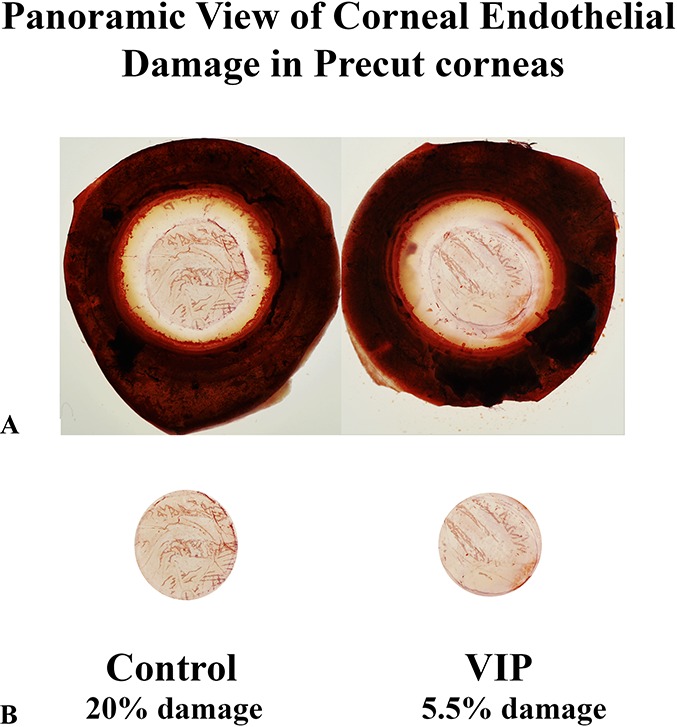
Beneficial effects of VIP treatment before precut on reducing central CE damage demonstrated in panoramic viewing of alizarin red-stained corneoscleral explants (endothelium side up) (18-year-old female donor with cancer of the brain). A, Whole corneoscleral explants. B, Central CE discs dissected photographically along the injury marks left by cornea precut.
DISCUSSION
The 2 CE cell autocrine factors, and CNTF, not only work as individual trophic factors but also have the capacity to work in concert for the establishment of a perpetual supply of endogenous VIP. Although VIP upholds the CNTFRα in the corneal endothelium and increases the responsiveness of the corneal endothelium to CNTF modulation,20 CNTF upregulates endogenous VIP expression in the corneal endothelium in human donor corneoscleral explants.15 The dramatic CE cell loss detected in 6 months after keratoplasty procedures may have resulted from the disruption of the CNTF/VIP signaling pathways. For instance, corneoscleral explants in storage gradually lose its CE CNTFRα.19 As such, CNTF upregulation of endogenous VIP expression in the corneal endothelium of the graft is likely not as effective as that in the intact eye. Treatment of the human donor corneoscleral explants with exogenous VIP before or in the middle of their storage (4°C) retards loss of CNTFRα from the corneal endothelium.20 The present study demonstrated in organ-cultured precut human donor corneoscleral explants that VIP treatment before precut resulted in retention of CNTFRα in the corneal endothelium (Fig. 3).
Is CNTF available in the recipient's anterior chamber to modulate the corneal endothelium of the graft? Although a definitive answer to the question awaits collection and analysis of the aqueous humor of the recipient eye at the time of surgery, indirect evidence suggests that the answer is yes. First of all, CNTF, which was discovered in an extract of the ciliary body, iris, and choroid,16 is an injury factor released only after injury through an unknown mechanism.31 Corneal transplant surgery-induced injury likely induces CNTF release from the recipient's ciliary body and iris to the anterior chamber of the recipient's eye. Second, the presence of CNTF in the aqueous humor of enucleated (injured) bovine eyes has been demonstrated32 and that of enucleated deceased donor eyes has been confirmed (data not shown).
CE N-cadherin and connexin 43 levels in precut corneas were the end points used in this study for demonstrating the treatment benefits of VIP. As a component of the adherens junctions,33 N-cadherin maintains the structural integrity of the corneal endothelium, as recently demonstrated in N-cadherin-knockout mice.14 In addition, N-cadherin as a mechanical sensor has been demonstrated in diverse experimental settings.34 Connexin 43 forms gap junctions in adjacent cells (allowing intercellular communication) and hemichannels in the unapposed cell membrane (allowing exchange of ions and signaling molecules between the cytoplasm and the extracellular milieu).34–36 Opening of the connexin 43 hemichannels is induced by mechanical stress through activation of integrin α5β137 and Akt kinase.38 Whether increased levels of these 2 mechanical sensors, N-cadherin and connexin 43, bring resilience to the corneal endothelium of the graft under mechanical force is not known at present. Future studies are warranted to investigate this possibility considering that folding and insertion of the graft at the time of surgery may have caused significant loss of CE cells.
Consistent with the effects of VIP treatments on upregulating the differentiation markers in the CE cells (Figs. 1–3), microscopic view of the trephined corneal endothelium revealed the effect of VIP treatment on reducing CE damage (revealed as areas of denuded, alizarin red-stained corneal endothelium) (Figs. 4 and 5). The beneficial effect of the VIP was confirmed in the panoramic view of the whole alizarin red S-stained corneal endothelium, in which the bias in choosing the field to photograph for image analysis was completely removed (Fig. 6).
VIP is a 28-amino acid peptide whose presence in aqueous humor has been demonstrated in the rabbit39 and the human.40 The levels of VIP in the aqueous humor collected during glaucoma surgery are significantly lower than during cataract surgery.40 Existence of glaucoma in recipients' eyes before penetrating keratoplasty is a clear risk factor for graft failure at 10 years after surgery.7 After keratoplasty procedures, the condition that exists in the glaucomatous eye, such as the low VIP level in the aqueous humor, may not be compatible with maintaining the differentiation state of the donor corneal endothelium of the graft.
Although the procedures of keratoplasty have been evolving, our understanding of the cause of the dramatic CE cell loss observed immediately after all types of keratoplasties has been lacking. Taking together the results we have gathered thus far, we predict that VIP treatment of the donor corneoscleral explants will sustain the differentiated state of the corneal endothelium of the graft in the recipient eyes, including those with glaucoma. Although it has failed the efficacy phase, safety of VIP administered intravenously or inhaled has been demonstrated in clinical trials of pulmonary arterial hypertension.41–44
CONCLUSIONS
Before cornea precut, treatment of human donor corneoscleral explants with CE cell autocrine VIP enhanced the differentiation state of CE grafts trephined from precut corneas.
Footnotes
Supported by National Institutes of Health (NIH) R21EY020595.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Stevenson W, Cheng SF, Dastjerdi MH, et al. Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (Lucentis) vs bevacizumab (Avastin). Ocul Surf. 2012;10:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuksel E, Yuksel N, Akata F. Comparison of two in situ corneal donation technique: morgue trephination or scleracorneal removal technique. Acta Ophthalmol. 2015;93:e573–e577. [DOI] [PubMed] [Google Scholar]
- 3.Lee WB, Jacobs DS, Musch DC, et al. Descemet's stripping endothelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:1818–1830. [DOI] [PubMed] [Google Scholar]
- 4.Terry MA, Shamie N, Chen ES, et al. Precut tissue for Descemet's stripping automated endothelial keratoplasty: vision, astigmatism, and endothelial survival. Ophthalmology. 2009;116:248–256. [DOI] [PubMed] [Google Scholar]
- 5.Terry MA, Shamie N, Straiko MD, et al. Endothelial keratoplasty: the relationship between donor tissue storage time and donor endothelial survival. Ophthalmology. 2011;118:36–40. [DOI] [PubMed] [Google Scholar]
- 6.Claerhout I, Beele H, Kestelyn P. Graft failure, I: endothelial cell loss. Int Ophthalmol. 2008;28:165–173. [DOI] [PubMed] [Google Scholar]
- 7.Sugar A, Gial RL, Kollman C, et al. Donor Study Writing Committee for the Cornea Donor Study Research Group. Factors associated with corneal graft survival in the cornea. JAMA Ophthalmol. 2015;133:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price MO, Bidros M, Gorovoy M, et al. Effect of incision width on graft survival and endothelial cell loss after Descemet stripping automated endothelial keratoplasty. Cornea. 2010;29:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh SW. CE autocrine trophic factor VIP in a mechanism-based strategy to enhance human donor cornea preservation for transplantation. Exp Eye Res. 2012;95:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh SW, Chandrasekara K, Abbondandolo CJ, et al. VIP and VIP gene silencing modulation of differentiation marker N-cadherin and cell shape of corneal endothelium in human corneas ex vivo. Invest Ophthalmol Vis Sci. 2008;49:3491–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakane F, Miyamoto Y. N-cadherin regulates the proliferation and differentiation of ventral midbrain dopaminergic progenitors. Dev Neurobiol. 2013;73:518–529. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto Y, Sakane F, Hashimoto K. N-cadherin-based adherens junction regulates the maintenance, proliferation, and differentiation of neural progenitor cells during development. Cell Adh Migr. 2015;9:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beebe DC, Coats JM. The lens organizes the anterior segment: specification of neural crest cell differentiation in the avian eye. Dev Biol. 2000;220:424–431. [DOI] [PubMed] [Google Scholar]
- 14.Vassilev VS, Mandai M, Yonemura S, et al. Loss of N-cadherin from the endothelium causes stromal edema and epithelial dysgenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 2012;53:7183–7193. [DOI] [PubMed] [Google Scholar]
- 15.Koh SWM, Guo Y, Bernstein SL, et al. Vasoactive intestinal peptide induction by ciliary neurotrophic factor in donor human corneal endothelium in situ. Neurosci Lett. 2007;423:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler R, Landa KB, Manthorpe M, et al. Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science. 1979;204:1434–1436. [DOI] [PubMed] [Google Scholar]
- 17.Koh SW. Ciliary neurotrophic factor released by corneal endothelium surviving oxidative stress ex vivo. Invest Ophthalmol Vis Sci. 2002;43:2887–2896. [PubMed] [Google Scholar]
- 18.Stockli KA, Lottspeich F, Sendtner M, et al. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989;342:920–923. [DOI] [PubMed] [Google Scholar]
- 19.Koh SW, Celeste J, Ku P. Functional CNTF receptor α subunit restored by its recombinant in CE cells in stored human donor corneas: connexin-43 upregulation. Invest Ophthalmol Vis Sci. 2009;50:1801–1807. [DOI] [PubMed] [Google Scholar]
- 20.Koh SW, Gloria D, Molloy J. CE autocrine VIP enhances its integrity in stored human donor corneoscleral explant. Invest Ophthalmol Vis Sci. 2011;52:5632–5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozog MA, Bernier SM, Bates DC, et al. The complex of ciliary neurotrophic factor-ciliary neurotrophic factor receptor alpha up-regulates connexin43 and intercellular coupling in astrocytes via the Janus tyrosine kinase/signal transducer and activator of transcription pathway. Mol Biol Cell. 2004;15:4761–4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le HT, Sin WC, Lozinsky S, et al. Gap junction intercellular communication mediated by connexin 43 in astrocytes is essential for their resistance to oxidative stress. J Biol Chem. 2014;289:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kar R, Riquelme MA, Werner S, et al. Connexin 43 channels protect osteocytes against oxidative stress-induced cell death. J Bone Miner Res. 2013;28:1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'hondt C, Iyyathurai J, Himpens B, et al. Cx43-hemichannel function and regulation in physiology and pathophysiology: insights from the bovine CE cell system and beyond. Front Physiol. 2014;5:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh SW, Cheng J, Dodson RM, et al. VIP down-regulates the inflammatory potential and promotes survival of dying (neural crest-derived) CE cells ex vivo: necrosis to apoptosis switch and up-regulation of Bcl-2 and N-cadherin. J Neurochem. 2009;109:792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh SW, Waschek JA. CE cell survival in organ cultures under acute oxidative stress: effect of VIP. Invest Ophthalmol Vis Sci. 2000;41:4085–4092. [PubMed] [Google Scholar]
- 27.Eye bank association of America. Eye banking statistical report. Available at: http://www.restoresight.org/wp-content/uploads/2015/03/2014_Statistical_Report-FINAL.pdf. Accessed March 2015.
- 28.Woodward MA, Titus M, Mavin K, et al. Corneal donor tissue preparation for endothelial keratoplasty. J Vis Exp. 2012;64:e3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saad HA, Terry MA, Shamie N, et al. An easy and inexpensive method for quantitative analysis of endothelial damage by using vital dye staining and Adobe Photoshop software. Cornea. 2008; 27:818–824. [DOI] [PubMed] [Google Scholar]
- 30.Panayotatos N, Everdeen D, Liten A, et al. Recombinant human CNTF receptor alpha: production, binding stoichiometry, and characterization of its activity as a diffusible factor. Biochemistry. 1994;33:5813–5818. [DOI] [PubMed] [Google Scholar]
- 31.Sendtner M, Stöckli KA, Thoenen H. Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration. J Cell Biol. 1992; 118:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh SWM. Vasoactive intestinal peptide acting in concert with ciliary neurotrophic factor to promote the survival of corneal endothelium under oxidative stress. In: Troger J, Kieselbach G, Bechrakis N. eds. Neuropeptides in the Eye. Kerala, India: Research Signpost; 2009:55–67. [Google Scholar]
- 33.Sun Z, Parrish AR, Hill MA, et al. N-cadherin, a vascular smooth muscle cell-cell adhesion molecule: function and signaling for vasomotor control. Microcirculation. 2014;21:208–218. [DOI] [PubMed] [Google Scholar]
- 34.Leckband DE, de Rooij J. Cadherin adhesion and mechanotransduction. Annu Rev Cell Dev Biol. 2014;30:291–315. See comment in PubMed Commons below. [DOI] [PubMed] [Google Scholar]
- 35.Wang T, Wang R, Cleary RA, et al. Recruitment of β-catenin to N-cadherin is necessary for smooth muscle contraction. J Biol Chem. 2015;290:8913–8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batra N, Kar R, Jiang JX. Gap junctions and hemichannels in signal transmission, function and development of bone. Biochim Biophys Acta. 2012;1818:1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batra N, Burra S, Siller-Jackson AJ, et al. Mechanical stress-activated integrin α5β1 induces opening of connexin 43 hemichannels. Proc Natl Acad Sci U S A. 2012;109:3359–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batra N, Riquelme MA, Burra S, et al. Direct regulation of osteocytic connexin 43 hemichannels through AKT kinase activated by mechanical stimulation. J Biol Chem. 2014;289:10582–10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor AW, Streilein JW, Cousins SW. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J Immunol. 1994;153:1080–1086. [PubMed] [Google Scholar]
- 40.Koh SW, Rutzen A, Coll T, et al. VIP immunoreactivity in human aqueous humor. Curr Eye Res. 2005;30:189–194. [DOI] [PubMed] [Google Scholar]
- 41.Said SI. Vasoactive intestinal peptide in pulmonary arterial hypertension. Am J Resp Crit Care. 2012;185:786. [DOI] [PubMed] [Google Scholar]
- 42.Gomberg-Maitland M, Bull TM, Saggar R, et al. New trial designs and potential therapies for pulmonary artery hypertension. J Am Coll Cardiol. 2013;62:D82–D91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma M, Pinnamaneni S, Aronow WS, et al. Existing drugs and agents under investigation for pulmonary arterial hypertension. Cardiol Rev. 2014;22:297–305. [DOI] [PubMed] [Google Scholar]
- 44.Lythgoe MP, Rhodes CJ, Ghataorhe P, et al. Why drugs fail in clinical trials in pulmonary arterial hypertension, and strategies to succeed in the future. Pharmacol Ther. 2016;164:195–203. [DOI] [PubMed] [Google Scholar]


