Abstract
Oligo-anuric individuals receiving hemodialysis (HD) are dependent on the dialysis machine to regulate sodium and water balance. Interest in adjusting the dialysate sodium concentration to promote tolerance of the HD procedure dates back to the early years of dialysis therapy. Evolution of dialysis equipment technologies and clinical characteristics of the dialysis population have prompted clinicians to increase the dialysate sodium concentration over time. Higher dialysate sodium concentrations generally promote hemodynamic stabilization and reduce intradialytic symptoms but often do so at the expense of stimulating thirst and promoting volume expansion. The opposite may be true for lower dialysate sodium concentrations. Observational data suggest that the association between dialysate sodium and outcomes may differ by serum sodium levels, supporting the trend toward individualization of the dialysate sodium prescription. However, lack of randomized controlled clinical trial data, along with operational safety concerns related to individualized dialysate sodium prescriptions, have prevented expert consensus regarding the optimal approach to the dialysate sodium prescription.
The kidneys play a central role in the homeostasis of the internal environment. In addition to toxin clearance, acid-base and electrolyte balance and vital enzyme and hormone production, the kidneys regulate sodium and water balance. Oligo-anuric individuals with end-stage kidney disease receiving hemodialysis (HD) depend on the dialysis machine to remove the sodium and water accumulated over the interdialytic interval and do so while maintaining a relatively constant plasma sodium concentration.1 The obvious exogenous source of sodium is dietary, but a less obvious source may be the dialysate fluid itself. While dietary sodium restrictions have been shown to reduce blood pressure (BP),2 adherence to such restrictions are difficult to achieve in clinical practice, and often are not sufficient in isolation to maintain normal BP. On the other hand, dialysate sodium is a readily modifiable aspect of the HD prescription and is a potential complement to dietary sodium restriction in the effort to achieve euvolemia and BP control among individuals receiving HD therapy. The importance of dialysis for volume control was realized early by the dialysis pioneer Belding Scribner who observed, “…hypertension appears to be influenced by the size of the extracellular space. The combination of dietary sodium restriction and ultrafiltration during dialysis permits regulation of extracellular volume.”3
Dialysate sodium prescriptions have evolved over the last 50 years with changes driven by technological advances and desire to improve the tolerability of the HD procedure. In the modern era, with its emphasis on efficiency and safe delivery of therapy to large populations, bulk-prepared dialysate has become commonplace. As a result, dialysate composition has become relatively standardized across facilities, particularly in the United States (U.S.). Increasing clinical and regulatory scrutiny of facility volume management practices has sparked renewed interest in defining the optimal approach to dialysate sodium prescription with growing attraction to tailoring the dialysate sodium concentration to individual patient needs. Higher dialysate sodium concentrations generally promote hemodynamic stabilization and reduce intradialytic symptoms but often do so at the expense of stimulating thirst and promoting volume expansion. On the other hand, lower dialysate sodium may lead to less thirst and associated weight gain, but at the expense of greater hemodynamic instability. Observational data suggest that the association between dialysate sodium and outcomes may differ by serum sodium levels, supporting the trend toward individualization of the dialysate sodium. However, lack of randomized controlled clinical trial data in this area has hindered development of clear clinical guidelines regarding the optimal approach to the dialysate sodium prescription.
Herein, we review the history of dialysate sodium titration, consider technical nuances of sodium measurement, summarize the pathophysiology and existing evidence linking dialysate sodium prescription to clinical outcomes and identify future research needs.
History of Dialysate Sodium Titration
Approach to dialysate sodium prescription: the early years
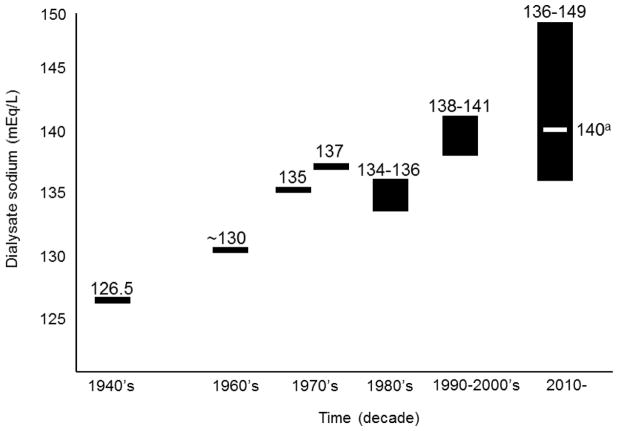
When dialysis was pioneered in the 1940’s, Dr. Willem Kolff set the dialysate sodium concentration to 126.5 mEq/L (lower than the patient’s serum sodium), recognizing the importance of diffusive sodium removal to thirst and BP control.4 Potential unfavorable hemodynamic consequences of such a low dialysate sodium concentration were offset by the high glucose concentration of the dialysate. Coil dialyzer membranes were unable to withstand high transmembrane pressures, so ultrafiltration was performed by osmosis. A supra-physiologic dialysate glucose concentration (>1,800 mg/dL)5 was used to generate an osmotic gradient for fluid removal.4 If an isonatremic dialysate had been used under these conditions, patients would have become hypernatremic. As such, typical dialysate sodium concentrations ranged from 126 to 130 mEq/L.5 The combined osmolar effect of the dialysate glucose, and to a lesser extent dialysate sodium, concentrations promoted hemodynamic stability during HD. The use of lower dialysate sodium concentrations remained commonplace through the 1960’s as administration of dialysis became more widespread.
Over time, as dialysis treatment times shortened, reports of headache, vomiting, blurred vision, tremors, seizures and disorientation began to accumulate in patients who were dialyzed with dialysate sodium concentrations <120 mEq/L.6 Collectively, these symptoms came to be known as the ‘dialysis disequilibrium syndrome’.7 As this symptom constellation was most commonly observed in patients initiating dialysis, several authorities attributed the syndrome to the combined effects of rapid volume removal and rapid shifts in plasma osmolality.8 Dialytic removal of urea as well as other osmotically-active molecules results in a decline of the extracellular osmolality relative to the intracellular osmolality. While urea is traditionally considered an ineffective osmole, very rapid clearance of urea by HD may generate a temporary, physiologically significant osmotic gradient between the intracellular and extracellular spaces.7, 9, 10 The pathophysiology of dialysis disequilibrium syndrome is not fully elucidated, but the syndrome’s clinical manifestations are largely attributable to cerebral edema.
These clinical observations, along with 1) the development of more resilient dialysis membranes, 2) introduction of hydrostatic ultrafiltration and 3) associated reduction in dialysate glucose concentrations, prompted use of higher dialysate sodium in subsequent years. By the 1960s, most facilities used a dialysate sodium concentration of 130 mEq/L.4 Figure 1 provides an overview of dialysate sodium titration over time.
Figure 1.
History of dialysate sodium titration.
aMost common facility dialysate sodium concentration.12
Recent trends in dialysate sodium prescription
During the 1970s and 1980s, dialysis technologies continued to evolve, allowing for even shorter treatment times. Secondarily, ultrafiltration rates increased and intradialytic hemodynamic instability became more common. Dialysate sodium concentrations were thus increased further to optimize intradialytic BP stability. By the 1980s the mean dialysate sodium concentration was 135 mEq/L.11 A decade later, the mean dialysate sodium had risen to 140 mEq/L, the most common concentration still today.12 To promote patient safety in hectic treatment environments, many facilities adopt facility-wide dialysate sodium prescriptions, standardizing prescriptions across all facility patients. In a 2011 study of almost 1,400 patients from a single dialysis provider, Munoz Mendoza et al. reported that over 50% of organization patients dialyzed with a dialysate sodium concentration of 140 mEq/L.12
Data from numerous international sources suggest that mean pre-dialysis serum sodium concentrations range 136–139 mmol/L.12, 13 Since dialysis patients in the U.S. have a median serum sodium of ~138 mmol/L,14 use of the typical dialysate sodium concentration of 140 mEq/L will result in net diffusive sodium gains among a majority of patients.
Most dialysis prescriptions utilize a constant dialysate sodium concentration throughout the HD treatment, but varying the dialysate sodium concentration during treatment can be employed to maximize gains from sodium’s osmotic properties while minimizing associated fluid retention. Such sodium profiling (or modeling) utilizes a higher dialysate sodium early in the treatment with progressive reduction over the course of dialysis, concluding treatment with a dialysate sodium concentration similar to or lower than plasma sodium levels. This approach promotes hemodynamic stabilization through a diffusive influx of sodium that corresponds to the timing of the rapid fall in plasma osmolality precipitated by removal of urea and other osmotically-active solutes early in the treatment. As the treatment progresses, the rapid decline in plasma osmolality abates and the dialysate sodium is concurrently lowered, which minimizes the development of hypertonicity and associated thirst and subsequent weight gains. In their 2011 study, Munoz-Mendoza et al. found that sodium modeling was ordered in over a third of HD prescriptions.12 However, most approaches to sodium modeling lead to ‘sodium loading’, as patients are exposed to a higher “time-averaged” dialysate sodium concentration of 140–145 mEq/L (the relevant value when considering sodium balance).15, 16 Thus, sodium modeling has generally fallen out of favor in recent years due to concerns about resultant volume expansion. As an alternative to sodium modeling and, as further reviewed below, some clinicians now individualize the dialysate sodium concentration by aligning dialysate sodium prescriptions with patient pre-dialysis serum sodium levels.
In the last 5 years, increasing recognition of volume control as a critical contributor to adverse outcomes among individuals receiving HD has heightened interest in dialysate sodium manipulation as a potential modifiable aspect of fluid management. However, despite the many changes to the dialysate sodium prescription over the years, there have been few randomized controlled clinical trials in this area, hindering consensus regarding the optimal approach to dialysate sodium prescription.
Dialysate Sodium in Practice
Preparation of the dialysate
In the modern era, dialysate is generated by mixing commercially-available, pre-formulated ‘acid’ and ‘bicarbonate’ concentrates. In the U.S. several different formulations with varying dialysate sodium concentrations are available. One approach to dialysate solution production requires on-site dialysate production with preparation and mixing of the individual acid and bicarbonate concentrates according to specific manufacturing guidelines. Another approach involves use of premixed acid and base concentrates (i.e. no need for onsite preparation). The HD machine proportioning system, the system responsible for the final dialysate composition delivered to the patient, combines these concentrated, premixed solutions with the water supply to generate the dialysate solution. Within the constraint of delivering an electro-neutral solution to the dialysis filter, treating physicians can further manipulate the dialysate composition by adjusting the relative dilutions of acid and bicarbonate concentrates.
Measurement of dialysate and serum sodium
First, it is important to understand the difference between sodium concentration and sodium activity, both of which can be measured in the dialysate and the blood. Sodium concentration refers to the number of sodium molecules present per unit of volume. Sodium activity refers to ionic activity (the number of sodium ions free in solution and thus available for diffusion or chemical reaction). Sodium concentration is greater than sodium activity in solutions where there are other constituents available for binding. Historically, flame photometry, a method that captures free and complexed sodium forms, was used to measure physiologic fluid sodium levels, and results were adjusted based on protein concentrations. Measurement source (blood vs. aqueous fluid) determined the need for result recalibration. Modern approaches to serum and dialysate sodium measurement utilize indirect and direct ion-sensitive electrodes (ISE), which measure sodium activity. Indirect ISE involves a dilution step based on the assumed aqueous proportion of the fluid being tested. In the case of dialysate, a protein-free liquid, the sodium activity generally approaches the sodium concentration. Direct ISE does not require a dilution step, and its results are typically ‘referenced’ to flame photometric standards for ease of interpretation.17, 18 Studies comparing the techniques of flame photometry, direct ISE and indirect ISE among HD patients are lacking. Some experts recommend direct ISE as the preferred method for dialysate sodium concentration measurement because it does not rely on dilution.19 However, in today’s practice, indirect ISE is most common in central laboratories.
The intradialytic environment is a dynamic one. During a dialysis treatment, blood composition and concentration constantly change, raising the potential for inaccuracies in dialysate sodium measurements made by indirect ISE. Furthermore, it is not practical to measure dialysate sodium by indirect ISE methods in real-time at each individual HD machine. Rather, as sodium is the predominant cation in dialysate, the dialysate conductivity can be measured as a surrogate for dialysate sodium concentration. The association between dialysate conductivity and dialysate sodium is relatively linear,20 such that a conductivity of 1 mS/cm is equivalent to a sodium concentration of 10 mEq/L in an aqueous (protein-free) solution.21 Therefore, when the dialysate sodium prescription is changed, it is actually the dialysate conductivity, not the dialysate sodium concentration per se, that is monitored and regulated.
Measured versus prescribed dialysate sodium
The assumption that the prescribed dialysate sodium is equivalent to the delivered (measured) dialysate sodium has been challenged in recent times. In a quality improvement project, Gul et al. analyzed the difference between measured and prescribed dialysate sodium across 333 HD treatments from four dialysis facilities.22 Two of the facilities performed weekly on-site dialysate mixing of the acid and base concentrates, and the other two facilities used pre-mixed acid concentrates and bicarbonate cartridges. Indirect ISE was used to measure the dialysate sodium. The authors found that, on average, 57% of measured dialysate sodium concentrations were within ± 2mmol/L of the prescribed dialysate sodium concentrations. However, this proportion varied widely, ranging from 25% to 77% across facilities. Dialysis facilities utilizing on-site acid and base concentrate mixing exhibited greater variability in the measured versus prescribed dialysate sodium. In general, a positive bias was observed: measured dialysate sodium tended to be higher than prescribed dialysate sodium.22 These findings, however, must be interpreted with caution as the facilities with greater variations in prescribed and delivered dialysate sodium were also the facilities utilizing individualized dialysate sodium concentrations. Disagreements between prescribed and delivered concentrations could thus be attributed to any one or some combination of 1) on-site mixing practices, 2) HD machine error or 3) human error.
This observation highlights important patient care and safety issues related to dialysate sodium prescription. Additionally, it underscores the importance of verifying the alignment of prescribed and measured dialysate sodium concentrations in future interventional studies of dialysate sodium.
There are several processing steps from which discrepancies between prescribed and delivered dialysate sodium concentrations can originate. First, individual manufacturers accept a specified margin of error (up to 2.5%) in acid and base concentrates.21 These small concentration differences can be magnified by the mixing procedures at dialysis facilities. One might assume that close monitoring of the dialysate conductivity by the HD machine and dialysis facility personnel might negate the influence of margin of error-level differences in concentrates. However, small margins of error are also accepted in conductivity monitoring. For example, conductivity alarms for some machines do not activate until conductivity reaches levels ± 0.5 mS/cm above theoretical conductivity (equivalent to dialysate sodium concentrations of ± 5 mEq/L).23 Furthermore, conductivity alarms require daily verification with calibration against standard solutions. Within these safeguards, there are also margins of error of up to ± 0.3 mS/cm, introducing another potential 2–3 mEq/L difference in the dialysate sodium concentration.22 These issues speak to the importance of strict attention to facility quality control protocols.
For the remainder of this review, we will assume that the delivered dialysate sodium is equal to the prescribed dialysate sodium. However, we caution the reader that this assumption cannot be made in clinical practice and therefore, all reported studies of dialysate sodium concentrations and outcomes must be viewed as potentially biased by unmeasured, and thus unaccounted for, differences in the prescribed and delivered dialysate sodium concentrations.
Rationale for Changing the Dialysate Sodium
Sodium removal during dialysis
During the dialysis procedure, sodium is lost via ultrafiltration (a convective process) and diffusion. Increased sodium removal can therefore be achieved by either increasing the ultrafiltration volume and/or by lowering the dialysate sodium concentration. For diffusive removal of sodium to occur, the dialysate sodium concentration must be less than the plasma concentration of sodium available for diffusion. There are several competing factors that determine the availablity of sodium for diffusion across the dialysis membrane. These include: 1) the sodium concentration in plasma water (which is greater than the total plasma concentration); 2) the reduction in sodium activity in plasma due to complexing of free sodium ions with other anions; and 3) the reduction in sodium activity in plasma due to the Gibbs-Donnan effect, which results from negatively charged plasma proteins that cannot diffuse across the membrane but complex with sodium. Therefore, in order for diffusive sodium removal to occur, it has been estimated that the dialysate sodium must be at least 2 mEq/L lower than the plasma sodium concentration.24, 25 Due to differences in membrane composition, plasma protein content and ultrafiltration volume, the Gibbs Donnan effect is variable and may be larger than predicted, further influencing sodium diffusion.16 Thus, even aligning the dialysate sodium concentration to the plasma sodium may result in a net positive sodium balance.
Dialytic sodium removal can be measured in the spent dialysate, but this is performed in research settings only. In clinical practice, measurement of the sodium removed during dialysis is indirect and partially reflected in changes in the serum sodium concentration immediately following dialysis. With time, as hypotonic fluid ingestion drives the serum sodium back toward a set-point, the serum sodium becomes less reflective of dialytic sodium removal. Similar to dialysate sodium measurements, serum sodium measurements are subject to variability, and acceptable margins of error have been established for the different sodium measurement techniques. Such variability should be considered when prescribing the dialysate sodium concentration.
Pathophysiology of dialysate sodium and clinical outcomes
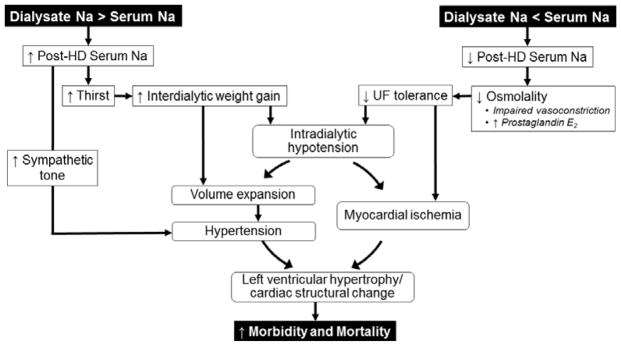
Figure 2 provides an overview of potential pathophysiologic pathways underlying associations between dialysate sodium concentrations and clinical outcomes. As dialysis technologies have evolved through the years, dialysate sodium concentrations have been adjusted to promote tolerability of the HD procedure. In the early years, dialysate sodium concentrations were manipulated to minimize rapid reductions in plasma osmolality and counteract symptoms associated with the dialysis disequilibrium syndrome. In more recent years, changes have targeted hemodynamic stability promotion with an eye toward minimizing weight gains and hypertension.
Figure 2.
Proposed pathophysiology underlying dialysate sodium and outcome associations.
When plasma osmolality rapidly drops during HD (as may occur in with the rapid removal of urea and other osmotically active molecules), plasma water moves into the relatively hyperosmolar intracellular compartment, leading to intravascular hypovolemia. This temporary decline in plasma osmolality also suppresses vasopressin release and promotes prostaglandin E2 release, impairing vasoconstriction and reducing vascular tone.26 When individuals are exposed to dialysate with sodium concentrations more than 2–3 mEq/L below plasma sodium concentrations, this drop in osmolality is amplified by the additional effect of sodium loss via diffusion (coupled with convective loss via ultrafiltration). When ultrafiltration outpaces plasma refill, and neural and cardiovascular compensatory responses are inadequate, BP falls. Intradialytic hypotension has been linked to transient myocardial ischemia as evidenced by elevated troponin T levels and episodes of myocardial “stunning” on transthoracic echocardiography studies.27–29 Animal studies suggest that repeat ischemic insults may lead to left ventricular hypertrophy and the downstream consequences of heart failure and arrhythmias.30, 31 Dialysis against a higher dialysate sodium concentration promotes hemodynamic stability by improving UF tolerance, both by increasing intravascular osmotic pressure and by improving vasoconstrictive compensatory responses.32
While higher dialysate sodium concentrations may have hemodynamic benefits, such benefits often come at the expense of volume expansion. When the dialysate sodium concentration exceeds the plasma sodium concentration and a patient is “sodium-loaded” during treatment, the thirst center is activated, leading to increased weight gains and subsequent volume expansion. Total body sodium balance also influences sympathetic tone and vasopressin release.8, 10 Data demonstrate that lower dialysate sodium concentrations can lower BP in absence of weight and serum sodium changes,33 suggesting that at least some of the net sodium loss occurs from non-dynamic sodium pools (e.g. the intracellular space).34 Dialysis against a lower dialysate sodium concentration thus may reduce hypertension and its cardiovascular sequelae through both volume-mediated and non-volume-mediated pathways, though it may prompt intradialytic hemodynamic instability in some cases.
Existing Evidence to Guide Selection of Dialysate Sodium Prescription
Evidence supporting use of higher dialysate sodium
Table 1 displays a summary of selected studies that provide support for potential benefits of higher dialysate sodium concentrations. Many of the early reports supporting the use of higher dialysate sodium focused on symptom outcomes such as muscle cramp frequency35, 36 and disequilibrium symptoms.37 A study from the mid-1980’s demonstrated an association between fixed higher dialysate sodium concentrations (144 vs. 133 mEq/L) and less cramping and fewer episodes of intradialytic hypotension.38 Studies of sodium modeling (vs. fixed sodium concentrations) also reported fewer cramps and less pre- to post-dialysis systolic BP decline.39–41 Overall, changes in dialysis technology and characteristics of the end-stage kidney disease population render these early studies poorly generalizable to modern practice.
Table 1.
Selected studies supporting the use of higher dialysate sodium.
| Author (Year) DNa Comparison | Patient and HD treatment characteristics | Study design | Outcomes | Comments | ||
|---|---|---|---|---|---|---|
| Pre-HD BP | IDWG | IDH and symptoms | ||||
| Early Studies, All Patients (No Mortality Data) | ||||||
|
Stewart (1972)35 145 vs. 132 mEq/L |
N=9 (960 tmts) -Twice weekly HD -Session length 16–22 h -Mean age NR |
Cross-over (2 arms) | NR | NR | ↓ cramping with higher DNa | -Small N -Twice weekly HD -Comorbidities unknown -BP and IDWG changes NR -Low sodium diet recommended but not evaluated -Acetate dialysate |
|
Port (1973)37 133 + hypertonic saline infusion vs. 133 mEq/L |
N=17 (22 tmts) -Incident and prevalent -Mean age 37y -Session length 4 h -Qb 250 mL/min -Qd 1000 mL/min |
Quasi-randomized (Attempte d to have similar pre-HD BUN in each group) |
↑ reduction in pre-HD SBP with lower DNa | ↓ IDWG with lower DNa | ↑ intradialytic BP with higher DNa ↑ disequilibrium symptoms with lower DNa ↑ frequency of EEG abnormalities with lower DNa |
-Small N -Comorbidities unknown -Acetate dialysate |
|
Dumler (1979)39 150 x 3h, then 130 x 1h vs. 140 mEq/L x 4h |
N=10 (2 wks/ arm) -Prevalent -Mean age 56y |
Double-blind cross-over (2 arms) | NS | NS | ↓ BP decline with higher DNa ↓ cramping with higher DNa |
-Small N -Comorbidities unknown -Acetate dialysate |
Raja (1983)40
|
N=10 (2 wks/ arm) -Prevalent -Mean age 61y |
Cross-over (4 arms); one week washout between each arm | NS | NS | ↑ IDH with DNa of 135 (A) and with DNa modeled 135→145 (D) | -Small N -Comorbidities unknown -Acetate dialysate |
|
Cybulsky (1985)38 144 vs. 133 mEq/L |
N=16 (24 wks/arm) -Prevalent -Mean age 46y -Non-diabetic |
Cross-over (2 arms) | NS | NS | ↓ IDH events in normotensive and anephric patients | -Small N -Heterogeneous sample with normotensive, hypertensive and anephric patients -Acetate dialysate |
|
Acchiardo (1991)41 149 →140 (linear) vs. 149 →140 (stepped) vs. 149 →140 (exponential) vs. 140 mEq/L (baseline) |
N=39 (9 weeks; arm chosen randomly each week and compared with baseline) -Prevalent -Mean age 49y |
Cross-over (3 arms) | NS | NS | ↓ IDH and cramping with modeled DNa | -Small N -TT 2 h -Bicarbonate dialysate -Comorbidities unknown |
| Hypotension Prone Patients (No Mortality Data) | ||||||
|
Levin (1996)44 Modeled 155–160→140 vs. fixed 140 mEq/L |
N=16 (3 wks/arm) -Prevalent -Mean age NR |
Double-blind cross-over (2 arms) | NS | NS | ↑ patient-reported preference for higher DNa | -Small N -Multiple comparisons -Intra-dialytic BP changes NR -Comorbidities unknown -Different starting DNa and UF protocols |
|
Sang (1997)42 140 vs. linear 155→140 vs. stepped 155→140 mEq/L |
N=23 (414 tmts; 2 wks/arm) -Prevalent -Mean age 59y |
Randomized, blinded cross-over (3 arms) | ↑ pre-HD BP with stepped modeling | ↑ IDWG with linear and stepped modeling | ↓ IDH with stepwise ↓ cramping with linear modeling ↑ thirst and fatigue with both stepped and linear |
-Small N -6 patients did not complete protocol and were excluded -Bicarbonate dialysate |
|
Oliver (2001)48 Modeled 152→142 +UF profiling versus 142 mEq/L + fixed UF rate |
N=33 (2 wks/arm) -Prevalent -Mean age~69y |
Randomized cross-over (2 arms) | NS | ↑ IDWG with higher DNa | ↓ symptoms with modeled DNa ↓ IDH with modeled DNa |
-Small N -Non-blinded -Protocol compared UF profiling versus fixed UF rates as well |
|
Song (2002)43 Combinations of fixed and modeled DNa with UF profiles vs. constant DNa 138 mEq/L |
N=11 (264 tmts; 33 sessions per arm) -Prevalent -Mean age 54y |
Randomized cross-over (8 arms) | NS | ↑ IDWG with sodium modeling | ↓ treatment failuresa with sodium modeling | -Small N -Complex protocol -Multiple comparisons |
| All patients (Mortality Data) | ||||||
|
Mc Causland (2012)14 >140 or modeled vs. ≤140 mEq/L |
N=2,272 -Thrice weekly HD -Prevalent -Mean age 62y |
Observational cohort | NS | ↑ IDWG with higher DNa | NR | -Higher Dialysate sodium associated with greater mortality in those with higher SNa |
|
Hecking (2012)13 >140 vs. 140 vs. <140 mEq/L |
N=11,555 -Thrice weekly HD -Prevalent -Mean age 62y |
Observati onal cohort | NR | NR | NR | -Excluded sodium modeling -Dialysate sodium >140 (vs. 140) associated with ↓ mortality among patients with pre-HD SNa <137 mmol/L |
|
Hecking (2012)46 >140 vs. 140 vs. <140 mEq/L |
N=23,593 -Thrice weekly HD -Prevalent -Mean age 63y |
Observational cohort | ↓ pre-HD BP with higher DNa | ↑ IDWG with higher DNa | NR | -DNa >140 associated with ↓ risk of hospitalization in full cohort -When restricted to facilities with standard (non-individualized) DNa, DNa >140 was associated with ↓ all-cause and CV-mortality |
Treatment failure defined as at least 1 of the following: HD treatment stopped before 75% of prescribed time completed; % UF achieved <70%; Kt/V <1.1.
Abbreviations: DNa, dialysate sodium; wks, weeks; tmt, treatment; HD, hemodialysis; BP, blood pressure; IDWG, interdialytic weight gain; NS, non-significant (p>0.05) difference; NR, not reported; CV, cardiovascular; BUN, blood urea nitrogen.
With time, physician-investigators found that a ‘one size fits all’ approach was not realistic due to variations in ultrafiltration tolerance and BP control across patients. Such recognition prompted the exploration of higher dialysate sodium use among patients prone to intradialytic hypotension (vs. all-comers as was studied in the earlier investigations). Several studies demonstrated associations between sodium modeling algorithms and fewer hypotensive episodes, but such hemodynamic benefits often occurred at the expense of greater thirst, weight gains and increased pre-HD BP.42–44 These studies had numerous weaknesses including small sample size, inclusion of multiple comparators and short durations. Additionally, they do not shed light on the independent contributions of interdialytic weight gains (IDWG) and extracellular volume status to outcomes.
Interdialytic weight gain is not an optimal surrogate for extracellular volume status. Individuals with lower IDWG may be volume-expanded post-dialysis if target weights are over-estimated. Likewise, individuals with larger IDWG may be volume-depleted post-dialysis if target weights are under-estimated. In both cases, extracellular volume status and IDWG are discordant and may independently influence outcomes.45
More recently, several large observational studies have considered dialysate sodium concentrations and mortality. Mc Causland et al. examined 2,272 patients from Satellite Healthcare and found that higher dialysate sodium concentrations (>140 mEq/L fixed or modeled vs. ≤140 mEq/L) were associated with greater mortality - but only among patients with higher pre-dialysis serum sodium levels. This finding was in spite of the fact that patients with lower serum sodium experienced modestly larger IDWGs.14
Using data from the more sizable Dialysis Outcomes and Practice Patterns Study (DOPPS) dataset, Hecking et al. reported that, among all patients, higher dialysate sodium concentrations were not associated with greater mortality but were associated with a lower risk of hospitalization (HR=0.97 per 2 mEq/L higher dialysate sodium, 95% CI 0.95–1.00, P=0.04). In an attempt to minimize confounding by prescribing patterns in relation to the mortality risk, they performed sensitivity analyses restricting the sample to facilities where more than 90% of patients had the same dialysate sodium; the adjusted HR for mortality remained lower (0.88 per 2 mEq/L decrease in dialysate sodium,95%CI 0.83–0.94).46 In a second analysis, and among patients with a lower pre-dialysis serum sodium, they reported a lower risk of mortality with the use of higher DNa.13 Together, these reports raise the possibility that there are select patients in whom the benefit of hemodynamic stabilization from higher dialysate sodium outweigh the potential downsides of modest increases in IDWG or BP.
Evidence supporting use of lower dialysate sodium
Table 2 displays a summary of selected studies that provide support for potential benefits of lower dialysate sodium concentrations. As outlined above, it soon became apparent that the hemodynamic benefits of higher dialysate sodium did not come without untoward consequences in some patients. Central to these side effects were the observations from early studies that patients tended to become thirstier and, consequently, had larger IDWG (and, in some cases, higher pre-dialysis BP).47, 48 In a more recent cohort of 30 Turkish HD patients, lower dialysate sodium concentration (137 vs. 143 mEq/L) was associated with greater brachial artery flow-mediated dilatation, smaller IDWG and lower mean 24 hour ambulatory BP (128/77 mmHg vs. 132/81 mmHg). However, these favorable findings came at the expense of greater intra-dialytic symptoms such as cramping and hypotension.49
Table 2.
Selected studies supporting the use of lower dialysate sodium.
| Author (Year) DNa Comparison | Patient and HD treatment characteristics | Study design | Outcome | Comments | ||
|---|---|---|---|---|---|---|
| Pre-HD BP | IDWG | IDH and symptoms | ||||
| All Patients | ||||||
|
Sadowski (1993)47 3 different modeling strategies of 148→138 vs. fixed 138 mEq/L |
N=16 (2 wks/arm) -Prevalent -Median age 19y |
Randomized cross-over (4 arms) | NS | ↑ IDWG with higher DNa | ↓ post-HD hypotension with higher DNa | -Small N -Younger, non-diabetic patients |
|
Thein (2007)50 141 vs. 138 mEq/L |
N=58 -Incident and prevalent -Mean age 52y |
Non-randomized facility level change in DNa (compared 4m pre- and 4m post-facility change) | ↓ BP with lower DNa | NS | NS | -10 patients dropped out during study period -Lack of detail on intra-dialytic symptoms and BP measurements -Non-randomized |
|
Davenport (2008)52 >140 vs. 140 vs. <140 mEq/L |
N=2,187 -Prevalent -Median age 61y |
Cross-sectional audit | ↑ BP with higher DNa | ↑ IDWG with higher DNa | ↑ IDH with higher DNa | -Cross-sectional -Potential confounding by indication for prescription of higher Dialysate sodium for hypotension-prone patients |
|
Kutlugün (2011)49 143 vs. 137 mEq/L |
N=30 (6 wks/arm) -Five patients on twice weekly HD -Prevalent -Mean age 48y |
Non-randomized cross-over (2 arms) | ↑ BP with higher DNa | ↑ IDWG with higher DNa | ↓ symptoms with higher DNa | -Small N -Inclusion of twice-weekly HD -Exclusion of hypotension-prone patients -Two patients withdrew due to symptoms on low DNa arm -Non-randomized |
| Hypotension Prone Patients | ||||||
|
Song (2002)43 Combinations of fixed and modeled DNa with UF profiles vs. constant DNa 138 mEq/L |
N=11 (264 tmts; 33 sessions per arm) -Prevalent -Mean age 54y |
Randomized cross-over (8 arms) | NS | ↑ IDWG with sodium modeling | ↓ treatment failures with sodium modelinga | -Small N -Complex protocol -Multiple comparisons |
Treatment failure defined as at least 1 of the following: HD treatment stopped before 75% of prescribed time completed; % ultrafiltration achieved <70%; Kt/V <1.1.
Abbreviations: Dialysate sodium, dialysate sodium; BP, blood pressure; IDH, intradialytic hypotension; NS, non-significant difference; NR, not reported; HD, hemodialysis.
Weight gain, volume expansion and BP change do not always go hand in hand.45 Thein et al. noted that a facility-wide decrease in dialysate sodium from 141 to 138 mEq/L was associated with a decrease in BP but found no change in IDWG.50 In fact, some studies have reported less frequent episodes of intradialytic hypotension with lower dialysate sodium. For example, in their crossover study of 27 patients, dePaula et al. noted that individualized dialysate sodium (restricted to patients whose pre-HD serum sodium was lower than 137 mmol/L) vs. fixed dialysate sodium of 138 mEq/L was associated with fewer intra-dialytic symptoms. It is important to point out that these patients were all non-diabetic and non-hypotension prone.51 In an audit of 2,187 British patients, Davenport et al. described less intradialytic hypotension with a dialysate sodium concentration 136–137 vs. ≥140 mEq/L.52 As this was a cross-sectional, clinical audit, it is likely that higher dialysate sodium concentrations were preferentially prescribed to hemodynamically unstable patients. Results must therefore be interpreted with caution as confounding by indication may introduce bias. To our knowledge, there have been no published studies showing an association between lower dialysate sodium concentrations and reduced mortality.
Individualization of the dialysate sodium or use of a “sodium alignment” protocol based on prior serum sodium measurements is attractive as it would reduce the diffusive sodium flux to the patient often associated with standardized dialysate sodium concentrations (typically 140 mEq/L) and protect against risk from exposure to overly low dialysate sodium concentrations. Relative stability of serum sodium levels over time, driven in part by tendency to return to an osmolar set point,16 provides some reassurance regarding the reliability of this approach. However, the only supportive data to-date come from small studies.51 Furthermore, current dialysis machines require that the dialysate sodium be re-set after each treatment. If the machine re-setting step is overlooked, subsequent patients would be dialyzed against dialysate sodium concentrations individualized to prior patients, potentially introducing risk.
Summary of Evidence
We have presented a summary of published reports that highlight potential benefits and drawbacks of the use of higher and lower dialysate sodium prescriptions. Over the last 50 years, the demographic make-up of the HD population and the technology driving the machines used to dialyze them have evolved considerably. Despite these advances and notwithstanding a greater appreciation of volume control as a component of dialysis adequacy, consensus regarding the optimal approach to dialysate sodium prescription for HD patients remains elusive.53–55 Indeed, a recent systematic review of 23 studies also arrived at this same conclusion.56 As in the past, and supported by the heterogeneity of the associations discussed above, a one-size-fits-all approach is likely not appropriate for the modern dialysate sodium prescription. It is likely (and perhaps probable) that the benefits of higher dialysate sodium outweigh the downsides for selected individuals, with the converse being true for lower dialysate sodium.
Evidence Gaps and Future Directions
In 2014, a coalition of dialysis organization leaders put forward a “Volume First” proposal that included a consensus opinion that intradialytic sodium loading should be avoided. They recommended prescribing dialysate sodium in the range of 134–138 mEq/L and using individualized dialysate sodium prescriptions for patients with relatively stable pre-dialysis serum sodium levels. They also called for dialysis machine manufacturers to develop machines that default to a standard dialysate sodium concentration between treatments. Finally, they advised against the use of hypertonic saline and sodium modeling.53 While there are data that support these recommendations, the evidence base is generally weak and contains no randomized controlled clinical trials. Furthermore, there are data of similar strength that suggest a survival advantage among patients dialyzed with higher dialysate sodium. Not surprisingly, others have urged caution in adopting these recommendations.55
To settle the debate regarding the optimal approach to the dialysate sodium prescription, randomized controlled clinical trials are needed. Thankfully there is room for optimism in this regard as we await the results of several ongoing studies (NCT02823821, NCT02145260 and ACTRN12611000975998). Future directions must include building on these on-going studies with larger, pragmatically-designed trials that evaluate the safety of administering individualized prescriptions to large populations in real-world treatment environments.
Additionally, consideration of whether the serum sodium is even the best reference on which to base individualization of dialysate sodium concentrations is warranted. In this regard, a recent study highlighted the association of calculated osmolality with greater intradialytic systolic BP decline. Mc Causland et al. reported independent associations between higher pre-dialysis serum urea nitrogen, higher serum glucose and lower serum sodium levels and greater intra-dialytic systolic BP declines.57 These findings suggest that hyponatremia may simply be a risk marker, rather than an independent risk factor, for hemodynamic instability among HD patients. It also suggests that there may be benefit to clinical interventions aimed at minimizing rapid plasma osmolality changes that do not require dialysate sodium manipulation and thus avoid the potential downsides of ‘sodium loading’ from higher dialysate sodium concentrations.
Conclusion
Volume management plays a key role in the morbidity and mortality experienced by individuals receiving HD therapy. Dialysate sodium concentration manipulation represents an appealing and underutilized aspect of the HD prescription with regard to the management of volume-related clinical issues. Considering the potential benefit of higher dialysate sodium concentrations among patients prone to experiencing hemodynamic instability or intradialytic symptoms and the potential benefit of lower dialysate sodium among patients prone to volume overload and hypertension, the optimal dialysate sodium concentration likely varies by person. Identifying the optimal approach to safe delivery of individualized dialysate sodium concentrations on a population level is an unmet dialysis delivery system need. Ongoing and future research efforts are urgently needed to address this most fundamental question in the safe and effective delivery of renal replacement therapy for our patients.
Acknowledgments
The authors thank Mary Katherine Huffman for her editorial assistance.
Footnotes
Disclosures
J.E.F. is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK109401 and has received speaking honoraria from Dialysis Clinic, Incorporated, Renal Ventures, American Renal Associates, American Society of Nephrology, Baxter and numerous universities and research funding for studies unrelated to dialysate sodium from the Renal Research Institute, a subsidiary of Fresenius Medical Care, North America. F.M.C is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K23DK102511 and has received consulting fees from GSK.
References
- 1.Peixoto AJ, Gowda N, Parikh CR, Santos SF. Long-term stability of serum sodium in hemodialysis patients. Blood Purif. 2010;29:264–267. doi: 10.1159/000274460. [DOI] [PubMed] [Google Scholar]
- 2.Charra B. Fluid balance, dry weight, and blood pressure in dialysis. Hemodial Int. 2007;11:21–31. doi: 10.1111/j.1542-4758.2007.00148.x. [DOI] [PubMed] [Google Scholar]
- 3.Scribner BH, Buri R, Caner JE, Hegstrom R, Burnell JM. The treatment of chronic uremia by means of intermittent hemodialysis: a preliminary report. Trans Am Soc Artif Intern Organs. 1960;6:114–122. [PubMed] [Google Scholar]
- 4.Drukker W, Parsons FM, Maher JF. Replacement of Renal Function by Dialysis: A Textbook of Dialysis. Boston, MA: Kluwer Boston Inc; 1983. [Google Scholar]
- 5.Sam R, Vaseemuddin M, Leong WH, Rogers BE, Kjellstrand CM, Ing TS. Composition and clinical use of hemodialysates. Hemodial Int. 2006;10:15–28. doi: 10.1111/j.1542-4758.2006.01170.x. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez FM, Pabico RC, Brown HW, Maher JF, Schreiner GE. Further experience with the use of routine intermittent hemodialysis in chronic renal failure. Trans Am Soc Artif Intern Organs. 1963;9:11–20. [PubMed] [Google Scholar]
- 7.Kennedy AC, Linton AL, Eaton JC. Urea levels in cerebrospinal fluid after haemodialysis. Lancet. 1962;1:410–411. doi: 10.1016/s0140-6736(62)91365-x. [DOI] [PubMed] [Google Scholar]
- 8.Flanigan M. Dialysate composition and hemodialysis hypertension. Semin Dial. 2004;17:279–283. doi: 10.1111/j.0894-0959.2004.17327.x. [DOI] [PubMed] [Google Scholar]
- 9.Dossetor JB, Oh JH, Dayes L, Pappius HM. Brain urea and water changes with rapid hemodialysis of uremic dogs. Trans Am Soc Artif Intern Organs. 1964;10:323–327. [PubMed] [Google Scholar]
- 10.Shimizu K, Kurosawa T, Ishikawa R, Sanjo T. Vasopressin secretion by hypertonic saline infusion during hemodialysis: effect of cardiopulmonary recirculation. Nephrol Dial Transplant. 2012;27:796–803. doi: 10.1093/ndt/gfr272. [DOI] [PubMed] [Google Scholar]
- 11.Munoz Mendoza J, Arramreddy R, Schiller B. Dialysate Sodium: Choosing the Optimal Hemodialysis Bath. Am J Kidney Dis. 2015;66:710–720. doi: 10.1053/j.ajkd.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Munoz Mendoza J, Sun S, Chertow GM, Moran J, Doss S, Schiller B. Dialysate sodium and sodium gradient in maintenance hemodialysis: a neglected sodium restriction approach? Nephrol Dial Transplant. 2011;26:1281–1287. doi: 10.1093/ndt/gfq807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hecking M, Karaboyas A, Saran R, Sen A, Hörl WH, Pisoni RL, Robinson BM, Sunder-Plassmann G, Port FK. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2012;59:238–248. doi: 10.1053/j.ajkd.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Mc Causland FR, Brunelli SM, Waikar SS. Dialysate sodium, serum sodium and mortality in maintenance hemodialysis. Nephrol Dial Transplant. 2012;27:1613–1618. doi: 10.1093/ndt/gfr497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambie SH, Taal MW, Fluck RJ, McIntyre CW. Online conductivity monitoring: validation and usefulness in a clinical trial of reduced dialysate conductivity. ASAIO J. 2005;51:70–76. doi: 10.1097/01.mat.0000150525.96413.aw. [DOI] [PubMed] [Google Scholar]
- 16.Flanigan MJ. Role of sodium in hemodialysis. Kidney Int Suppl. 2000;76:S72–78. doi: 10.1046/j.1523-1755.2000.07609.x. [DOI] [PubMed] [Google Scholar]
- 17.Levy GB. Determination of sodium with ion-selective electrodes. Clin Chem. 1981;27:1435–1438. [PubMed] [Google Scholar]
- 18.Burnett RW, Covington AK, Fogh-Andersen N, Külpmann WR, Lewenstam A, Maas AH, Müller-Plathe O, Sachs C, Siggaard-Andersen O, VanKessel AL, Zijlstra WG. Recommendations for measurement of and conventions for reporting sodium and potassium by ion-selective electrodes in undiluted serum, plasma or whole blood. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). IFCC Scientific Division Working Group on Selective Electrodes. Clin Chem Lab Med. 2000;38:1065–1071. doi: 10.1515/CCLM.2000.159. [DOI] [PubMed] [Google Scholar]
- 19.Santos SF, Peixoto AJ. Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:522–530. doi: 10.2215/CJN.03360807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locatelli F, Di Filippo S, Manzoni C. Relevance of the conductivity kinetic model in the control of sodium pool. Kidney Int Suppl. 2000;76:S89–95. doi: 10.1046/j.1523-1755.2000.07611.x. [DOI] [PubMed] [Google Scholar]
- 21.Courivaud C, Davenport A. Measurement and interpretation of serum sodium in end-stage kidney disease patients. Semin Dial. 2014;27:542–544. doi: 10.1111/sdi.12265. [DOI] [PubMed] [Google Scholar]
- 22.Gul A, Miskulin DC, Paine SS, Narsipur SS, Arbeit LA, Harford AM, Weiner DE, Schrader R, Horowitz BL, Zager PG. Comparison of Prescribed and Measured Dialysate Sodium: A Quality Improvement Project. Am J Kidney Dis. 2016;67:439–445. doi: 10.1053/j.ajkd.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Fresenius Medical Care. Hemodialysis Machine Operator’s Manual. Fresenius Medical Care-North America; 2008K. http://www.freseniusmedicalcare.us/fileadmin/data/us/pdf/HealthCareProfessionals/Renal_Products/Dialysis/PS_Documentation/03_Operators_manuals/490042_Rev_O.pdf. [Google Scholar]
- 24.Locatelli F, Ponti R, Pedrini L, Costanzo R, Di Filippo S, Marai P, Pozzi C. Sodium kinetics across dialysis membranes. Nephron. 1984;38:174–177. doi: 10.1159/000183303. [DOI] [PubMed] [Google Scholar]
- 25.Kooman JP, van der Sande F, Leunissen K, Locatelli F. Sodium balance in hemodialysis therapy. Semin Dial. 2003;16:351–355. doi: 10.1046/j.1525-139x.2003.16070.x. [DOI] [PubMed] [Google Scholar]
- 26.Schultze G, Maiga M, Neumayer HH, Wagner K, Keller F, Molzahn M, Nigam S. Prostaglandin E2 promotes hypotension on low-sodium hemodialysis. Nephron. 1984;37:250–256. doi: 10.1159/000183259. [DOI] [PubMed] [Google Scholar]
- 27.Selby NM, Fluck RJ, Taal MW, McIntyre CW. Effects of acetate-free double-chamber hemodiafiltration and standard dialysis on systemic hemodynamics and troponin T levels. ASAIO J. 2006;52:62–69. doi: 10.1097/01.mat.0000189725.93808.58. [DOI] [PubMed] [Google Scholar]
- 28.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4:1925–1931. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe N. Left ventricular remodeling: pathophysiology and treatment. Heart Fail Monit. 2003;4:55–61. [PubMed] [Google Scholar]
- 31.Ritz E, Wanner C. The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol. 2008;3:920–929. doi: 10.2215/CJN.04571007. [DOI] [PubMed] [Google Scholar]
- 32.Shimizu K, Kurosawa T, Sanjo T. Effect of hyperosmolality on vasopressin secretion in intradialytic hypotension: a mechanistic study. Am J Kidney Dis. 2008;52:294–304. doi: 10.1053/j.ajkd.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 33.Flanigan MJ, Khairullah QT, Lim VS. Dialysate sodium delivery can alter chronic blood pressure management. Am J Kidney Dis. 1997;29:383–391. doi: 10.1016/s0272-6386(97)90199-2. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen MK, Kurtz I. Are the total exchangeable sodium, total exchangeable potassium and total body water the only determinants of the plasma water sodium concentration? Nephrol Dial Transplant. 2003;18:1266–1271. doi: 10.1093/ndt/gfg112. [DOI] [PubMed] [Google Scholar]
- 35.Stewart WK, Fleming LW, Manuel MA. Muscle cramps during maintenance haemodialysis. Lancet. 1972;1:1049–1051. doi: 10.1016/s0140-6736(72)91224-x. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson R, Barber SG, Robson V. Cramps, thirst and hypertension in hemodialysis patients -- the influence of dialyzate sodium concentration. Clin Nephrol. 1977;7:101–105. [PubMed] [Google Scholar]
- 37.Port FK, Johnson WJ, Klass DW. Prevention of dialysis disequilibrium syndrome by use of high sodium concentration in the dialysate. Kidney Int. 1973;3:327–333. doi: 10.1038/ki.1973.51. [DOI] [PubMed] [Google Scholar]
- 38.Cybulsky AV, Matni A, Hollomby DJ. Effects of high sodium dialysate during maintenance hemodialysis. Nephron. 1985;41:57–61. doi: 10.1159/000183547. [DOI] [PubMed] [Google Scholar]
- 39.Dumler F, Grondin G, Levin NW. Sequential high/low sodium hemodialysis: an alternative to ultrafiltration. Trans Am Soc Artif Intern Organs. 1979;25:351–353. [PubMed] [Google Scholar]
- 40.Raja R, Kramer M, Barber K, Chen S. Sequential changes in dialysate sodium (DNa) during hemodialysis. Trans Am Soc Artif Intern Organs. 1983;29:649–651. [PubMed] [Google Scholar]
- 41.Acchiardo SR, Hayden AJ. Is Na+ modeling necessary in high flux dialysis? ASAIO Trans. 1991;37:M135–137. [PubMed] [Google Scholar]
- 42.Sang GL, Kovithavongs C, Ulan R, Kjellstrand CM. Sodium ramping in hemodialysis: a study of beneficial and adverse effects. Am J Kidney Dis. 1997;29:669–677. doi: 10.1016/s0272-6386(97)90118-9. [DOI] [PubMed] [Google Scholar]
- 43.Song JH, Lee SW, Suh CK, Kim MJ. Time-averaged concentration of dialysate sodium relates with sodium load and interdialytic weight gain during sodium-profiling hemodialysis. Am J Kidney Dis. 2002;40:291–301. doi: 10.1053/ajkd.2002.34507. [DOI] [PubMed] [Google Scholar]
- 44.Levin A, Goldstein MB. The benefits and side effects of ramped hypertonic sodium dialysis. J Am Soc Nephrol. 1996;7:242–246. doi: 10.1681/ASN.V72242. [DOI] [PubMed] [Google Scholar]
- 45.Hecking M, Karaboyas A, Antlanger M, Saran R, Wizemann V, Chazot C, Rayner H, Hörl WH, Pisoni RL, Robinson BM, Sunder-Plassmann G, Moissl U, Kotanko P, Levin NW, Säemann MD, Kalantar-Zadeh K, Port FK, Wabel P. Significance of interdialytic weight gain versus chronic volume overload: consensus opinion. Am J Nephrol. 2013;38:78–90. doi: 10.1159/000353104. [DOI] [PubMed] [Google Scholar]
- 46.Hecking M, Karaboyas A, Saran R, Sen A, Inaba M, Rayner H, Hörl WH, Pisoni RL, Robinson BM, Sunder-Plassmann G, Port FK. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol. 2012;7:92–100. doi: 10.2215/CJN.05440611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadowski RH, Allred EN, Jabs K. Sodium modeling ameliorates intradialytic and interdialytic symptoms in young hemodialysis patients. J Am Soc Nephrol. 1993;4:1192–1198. doi: 10.1681/ASN.V451192. [DOI] [PubMed] [Google Scholar]
- 48.Oliver MJ, Edwards LJ, Churchill DN. Impact of sodium and ultrafiltration profiling on hemodialysis-related symptoms. J Am Soc Nephrol. 2001;12:151–156. doi: 10.1681/ASN.V121151. [DOI] [PubMed] [Google Scholar]
- 49.Aybal Kutlugüns A, Erdem Y, Okutucu S, Yorgun H, Atalar E, Arici M. Effects of lowering dialysate sodium on flow-mediated dilatation in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26:3678–3682. doi: 10.1093/ndt/gfr092. [DOI] [PubMed] [Google Scholar]
- 50.Thein H, Haloob I, Marshall MR. Associations of a facility level decrease in dialysate sodium concentration with blood pressure and interdialytic weight gain. Nephrol Dial Transplant. 2007;22:2630–2639. doi: 10.1093/ndt/gfm220. [DOI] [PubMed] [Google Scholar]
- 51.de Paula FM, Peixoto AJ, Pinto LV, Dorigo D, Patricio PJ, Santos SF. Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int. 2004;66:1232–1238. doi: 10.1111/j.1523-1755.2004.00876.x. [DOI] [PubMed] [Google Scholar]
- 52.Davenport A, Cox C, Thuraisingham R Group, PRA. The importance of dialysate sodium concentration in determining interdialytic weight gains in chronic hemodialysis patients: the PanThames Renal Audit. Int J Artif Organs. 2008;31:411–417. doi: 10.1177/039139880803100506. [DOI] [PubMed] [Google Scholar]
- 53.Weiner DE, Brunelli SM, Hunt A, Schiller B, Glassock R, Maddux FW, Johnson D, Parker T, Nissenson A. Improving clinical outcomes among hemodialysis patients: a proposal for a “volume first” approach from the chief medical officers of US dialysis providers. Am J Kidney Dis. 2014;64:685–695. doi: 10.1053/j.ajkd.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Port F, Hecking M, Karaboyas A, Pisoni R, Robinson B. Current evidence argues against lowering the dialysate sodium. Nephrol News Issues. 2013;27:18–21. [PubMed] [Google Scholar]
- 55.Hecking M, Rayner H, Port FK. More evidence needed before lower dialysate sodium concentrations can be recommended. Am J Kidney Dis. 2015;65:519–520. doi: 10.1053/j.ajkd.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 56.Basile C, Pisano A, Lisi P, Rossi L, Lomonte C, Bolignano D. High versus low dialysate sodium concentration in chronic haemodialysis patients: a systematic review of 23 studies. Nephrol Dial Transplant. 2016;31:548–563. doi: 10.1093/ndt/gfv084. [DOI] [PubMed] [Google Scholar]
- 57.Mc Causland FR, Waikar SS. Association of Predialysis Calculated Plasma Osmolarity With Intradialytic Blood Pressure Decline. Am J Kidney Dis. 2015;66:499–506. doi: 10.1053/j.ajkd.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]