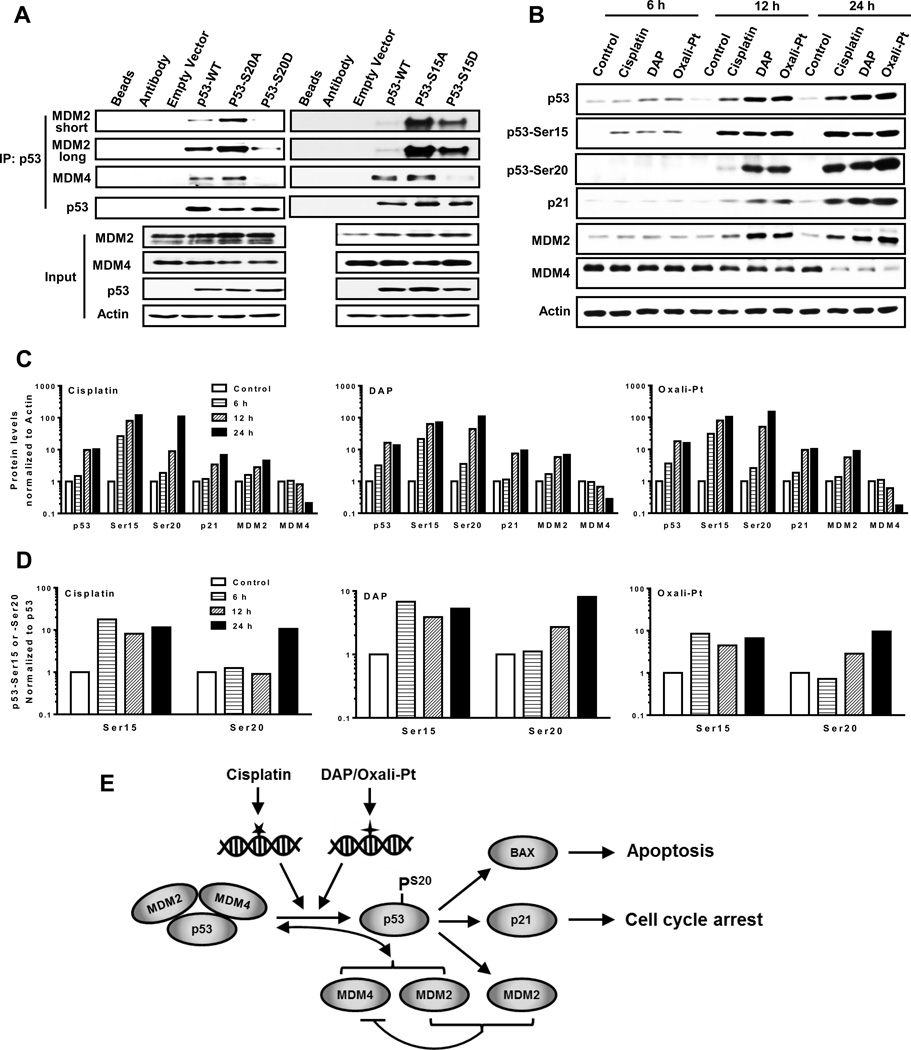
Figure 7.
The effect of Ser15 and Ser20 phosphorylation on MDM2 and MDM4 binding to p53. (A) A2780 p53−/− cells were transfected with empty vector, wild type p53 (p53-WT) and p53 mutant S20A, S20D, S15A or S15D for 48 hours. Cell lysates were then prepared and immunoprecipitated with the p53 antibody (FL-393), the proteins resolved by SDS-PAGE and immunoblotted with the indicated antibodies (DO-7 for p53). Whole cell lysates were used as input. (B) A2780 cells treated with cisplatin, DAP or oxaliplatin were harvested after 6, 12 or 24 hr treatment. Cell lysates were then prepared and subjected to Western blot analysis using the indicated antibodies. The signals from immunoblots in (B) were quantified by densitometry, and the ratios of indicated proteins to β-Actin were normalized to control and plotted for each platinum drug (C). The ratios of Ser15:total p53 and Ser20:total p53 were also determined and plotted similarly (D). (E) Schematic representation and summary of the distinct DNA damage induced by cisplatin and DAP or oxaliplatin that result in activation of independent pathways for phosphorylating p53 at Ser20, induction of MDM4 degradation and disruption of the p53-MDM2-MDM4 tri-protein complex in sensitive tumor cells. In resistant 2780CP/Cl-16 and 2780CP/Cl-24 cells, however, Ser20 phosphorylation by cisplatin is absent, and this disrupts normal p53 function and induces resistance. DAP and oxaliplatin, by virtue of their ability to use an independent pathway to restore Ser20 phosphorylation, activate p53 function and circumvent cisplatin resistance. Transactivation of MDM2 is important for inducing degradation of MDM4 in order to prevent re-formation of the inhibitory p53-MDM2-MDM4 complex and this, therefore, may prolong p53 activity. The stoichiometry of this complex is unknown and is presented here as a 1:1:1 complex for demonstration purposes only.