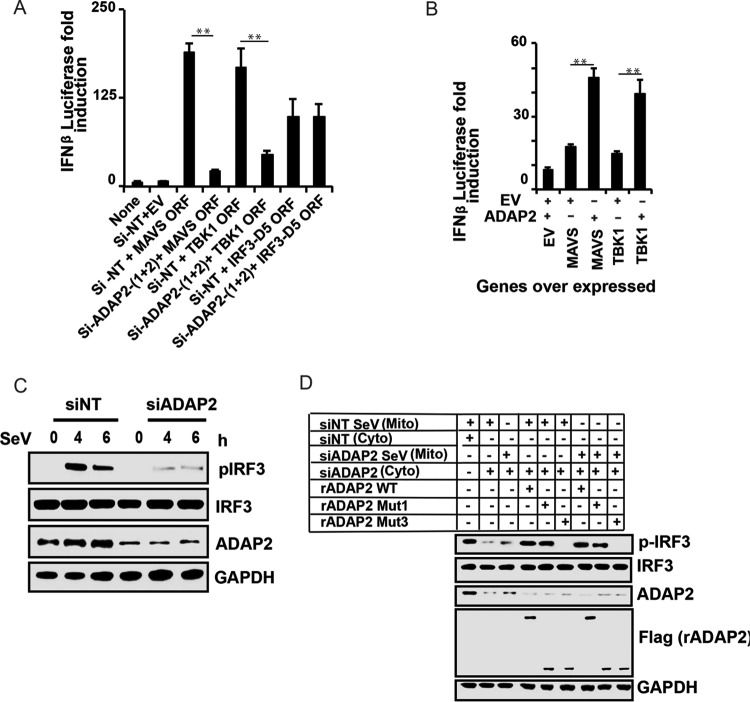
FIG 3.
ADAP2 is needed for IRF3 phosphorylation. (A) Knockdown of ADAP2 reduced IFN-β promoter-driven luciferase reporter activity induced by ectopic expression of MAVS, TBK1, and IRF3-D5 in HEK293T cells. (B) Coexpression of ADAP2 increased IFN-β promoter-driven luciferase reporter activity induced by overexpression of MAVS and TBK1. (C) ADAP2 silencing reduced SeV infection-induced IRF3 phosphorylation in HEK293T cells. (D) A cell-free in vitro RIG-I-mediated IRF3 phosphorylation assay established ADAP2 as a regulator of the interferon response. Western blotting was used to detect pIRF3 formation after stimulation of uninfected HEK293T cellular cytoplasm with mitochondria from infected HEK293T cells. The ADAP2-silenced cytoplasm was supplemented with either vehicle only or recombinant ADAP2 (full length, Mut1, and/or Mut3). Reporter (firefly) luciferase values were normalized with a renilla luciferase internal control and expressed as fold induction values. The values shown are means and SD for a representative experiment performed in triplicate. The statistical significance of differences in mean values was analyzed using unpaired two-tailed Student's t test, and P values of <0.05 were considered statistically significant (**, P < 0.01). Si, siRNA; Si-NT, nontargeting negative-control siRNA; EV, empty vector; ORF, open reading frame; h, hours postinfection; none, only the IFN-β promoter-driven luciferase reporter was present within the cells; mito, mitochondrial fraction; cyto, cytoplasmic fraction; rADAP2, purified recombinant ADAP2 protein; WT, wild-type full-length ADAP2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase (cytoplasmic internal control marker); siNT Sev (Mito), mitochondrial fraction of siNT-treated Sendai virus-infected cells; siNT (Cyto), cytoplasmic fraction of siNT-treated uninfected cells; siADAP2 Sev (Mito), mitochondrial fraction of siADAP2-treated Sendai virus-infected cells; siADAP2 (Cyto), cytoplasmic fraction of siADAP2-treated uninfected cells.