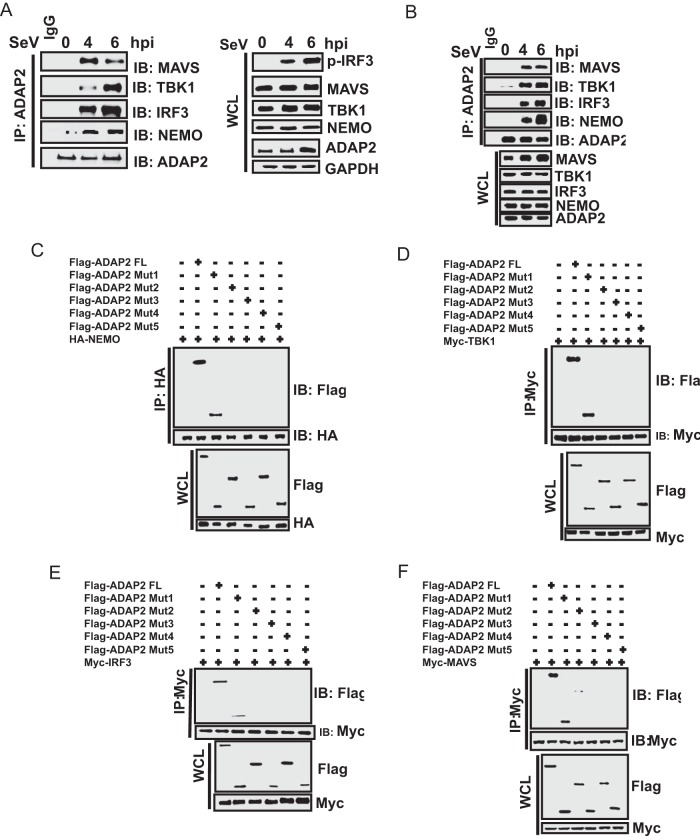
FIG 4.
The ArfGAP domain of ADAP2 interacts with RIG-I pathway proteins. Cells were infected with SeV, and endogenous protein interactions were detected using coimmunoprecipitation and Western blotting. (A) Immunoprecipitated ADAP2 from HEK293T cells showed interactions with MAVS, NEMO, TBK1, and IRF3 upon SeV infection. (B) Immunoprecipitated ADAP2 from human primary monocytes showed interactions with MAVS, NEMO, TBK1, and IRF3 upon SeV infection. (C to F) Coimmunoprecipitation of ectopically expressed full-length NEMO (C), TBK1 (D), IRF3 (E), and MAVS (F) in cells transfected with various truncation plasmids of ADAP2 identified that the ArfGAP domain interacts with RIG-I pathway proteins. hpi, hours postinfection; FL, full length; Mut, truncation mutant of ADAP2; SeV, Sendai virus; WCL, whole-cell lysate; IP, immunoprecipitation; IB, immunoblot; HA, hemagglutinin tag; GAPDH, glyceraldehyde-3-phosphate dehydrogenase (cytoplasmic internal control marker).