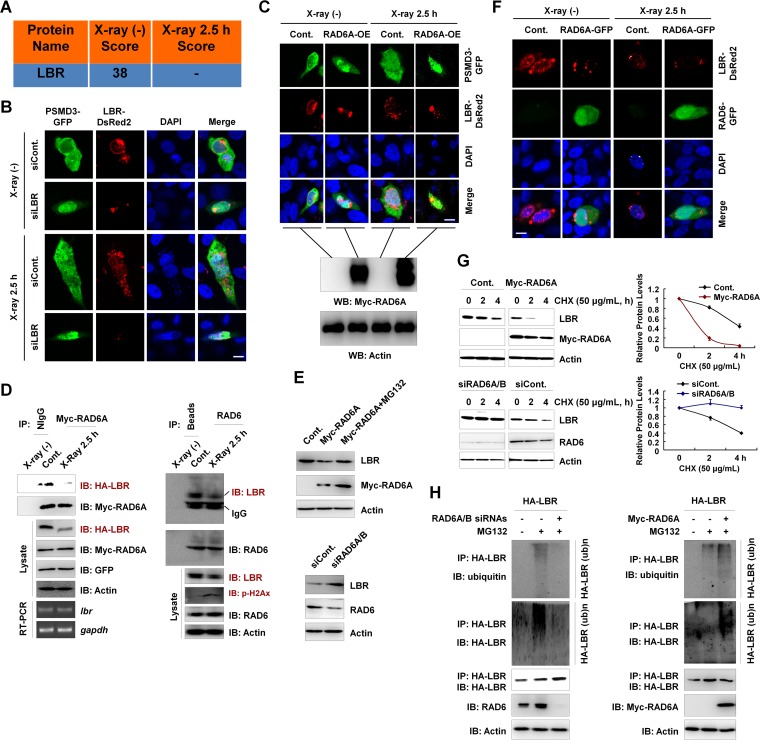
FIG 5.
LBR regulates the nuclear localization of proteasomes, and the degradation of LBR is affected by RAD6. (A) LBR is a potential RAD6-interacting protein, as indicated in our mass spectrometry analysis in Fig. 1. The score refers to the obtained value analyzed by Mascot software on the basis of the original mass spectrum data. (B) Knockdown of LBR results in the nuclear translocation of proteasomes, as indicated by PSMD3 immunostaining. HEK293T cells were transfected with a GFP-tagged PSMD3 plasmid and a DsRed2-tagged LBR together with a control siRNA (siCont) or an LBR-specific siRNA (siLBR), as indicated, for 48 h. Cells were irradiated with X rays for 2.5 h or not irradiated. Cells were then subjected to immunofluorescence assays by confocal laser microscopy. DAPI (4′,6-diamidino-2-phenylindole) staining was used to indicate the cell nucleus. Bar, 10 μm. (C) RAD6 overexpression promotes the nuclear translocation of proteasomes. HEK293T cells stably expressing a Myc-tagged RAD6A plasmid were transfected with GFP-tagged PSMD3 to indicate the proteasomes and DsRed2-tagged LBR for 48 h. Cells were irradiated with X rays for 2.5 h or not irradiated. (Top) The cell nucleus is indicated by DAPI staining. Cells were then subjected to immunofluorescence assays by confocal laser microscopy. Bar, 10 μm. (Bottom) The results of validation of Myc-RAD6A overexpression by Western blotting (WB). (D) RAD6 interacts with LBR, and X-ray irradiation downregulates LBR protein levels. Myc-tagged RAD6A and HA-tagged LBR were cotransfected into HEK293T cells for 48 h. Cells were then subjected to X-ray irradiation (X-ray 2.5 h) or not irradiated (control) as indicated. Cell extracts were prepared and subjected to co-IP analyses with anti-Myc antibodies, and Western blot assays were performed with the indicated antibodies. Reverse transcription-PCR (RT-PCR) assays were also performed using cells treated similarly. The detected genes are indicated (left). gapdh, glyceraldehyde-3-phosphate dehydrogenase gene. For endogenous co-IP assays, HEK293T cells were harvested and subjected to co-IP assays with anti-RAD6 antibodies, followed by Western blot analyses with the indicated antibodies (right). (E) RAD6 controls LBR protein levels in a proteasome-dependent manner. HEK293T cells transfected with or without (control) a Myc-tagged RAD6A plasmid were treated with MG132 for 8 h or not treated, as indicated. (Top) Cell extracts were prepared and subjected to Western blot analyses with the indicated antibodies. (Bottom) Cells transfected with a control siRNA or RAD6A/B-specific siRNAs (siRAD6A/B) were lysed and subjected to Western blot assays with the indicated antibodies. (F) Immunostaining assays support the conclusion that RAD6 regulates LBR protein levels. HEK293T cells were transfected with an equal amount of DsRed2-tagged LBR together with or without (control) a GFP-tagged RAD6A plasmid for 48 h. Cells were then irradiated with X rays for 2.5 h or not. Cells were subjected to immunofluorescence assays by confocal laser microscopy. DAPI staining was used to indicate the cell nucleus. Bar, 10 μm. (G) RAD6 regulates the degradation of LBR. (Top) Cells transfected with an empty vector expressing Myc or Myc-tagged RAD6A were treated with 50 μg/ml cycloheximide (CHX) for the indicated times. (Left) Cells were then harvested and subjected to Western blot analyses with the indicated antibodies. (Right) The bands were quantified, and the bars indicate the standard deviations from three biological replicates. (Bottom) Cells transfected with a control siRNA or RAD6A/B-specific siRNAs were treated with 50 μg/ml CHX for the indicated times. (Left) Cells were then harvested and subjected to Western blot analyses with the indicated antibodies. (Right) The bands were quantified, and the bars indicate the standard deviations from three biological replicates. (H) RAD6 regulates the ubiquitination of LBR. (Left) HEK293T cells expressing HA-tagged LBR were transfected with a control siRNA (−) or RAD6A/B-specific siRNAs (+) for 48 h, and cells were treated with MG132 for 8 h or not treated, as indicated. Immunoprecipitation assays were performed under denaturing conditions with anti-HA antibodies, followed by Western blot analyses with the indicated antibodies. (Right) HEK293T cells were transfected with the HA-tagged LBR plasmid with or without a Myc-tagged RAD6A plasmid for 48 h, and cells were treated with MG132 for 8 h or not treated, as indicated. Immunoprecipitation assays were performed under denaturing conditions with anti-HA antibodies, followed by Western blot analyses with the indicated antibodies.