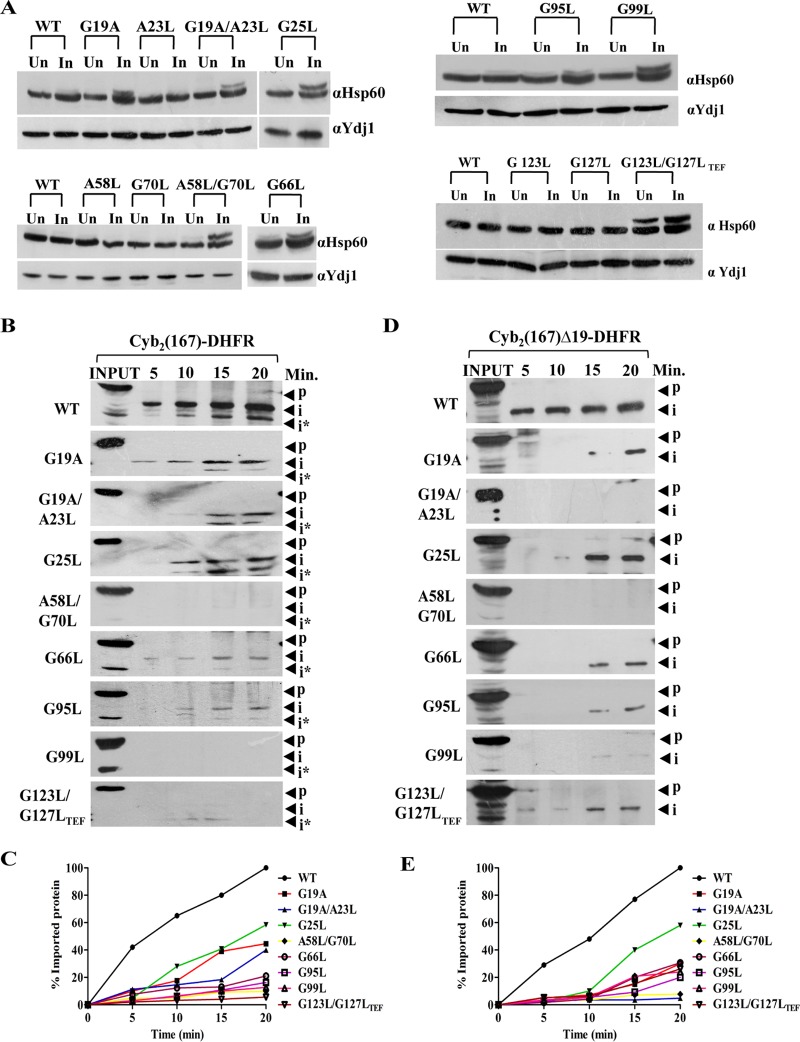
FIG 4.
Critical role of Tim17 in regulating both matrix sorting and inner membrane sorting. (A) In vivo precursor accumulation assay. Wild-type and tim17 mutant cells were grown to early log phase and subjected to heat shock at 37°C for 4 h. Cell lysates were prepared and analyzed by Western blotting using Hsp60-specific antibodies; Un, uninduced; In, induced. (B) In vitro import kinetic analysis. Purified mitochondria from wild-type and different mutant yeast strains were subjected to heat shock at 37°C for 30 min to induce phenotype, followed by incubation with saturating amounts of purified cytb2 (1–167)-DHFR at 25°C. The reaction was terminated by the addition of valinomycin at different time intervals; aliquots were subjected to proteinase K treatment. The samples were subsequently analyzed by Western blotting using an anti-DHFR antibody; p, precursor; i and i*, intermediates. (C) The signal intensity of imported intermediate bands obtained from Western blots was quantified by densitometry using ImageJ software for cytb2 (1–167)-DHFR after normalizing the amounts of precursor imported in the wild type at 20 min as 100% import. (D and E) Mitochondria from wild-type and tim17 mutant cells were subjected to heat shock at 37°C for 30 min to induce phenotype followed by incubation with saturating amounts of purified cytb2 (1–167)Δ19-DHFR at 25°C. The import reaction was terminated by valinomycin at indicated time intervals, followed by treatment with proteinase K. The samples were subsequently analyzed by Western blotting using an anti-DHFR antibody; p, precursor; i, intermediate (D). The imported intermediate bands were quantified by densitometry using ImageJ software after normalizing the amounts of precursor imported in the wild type at 20 min as 100% import (E).