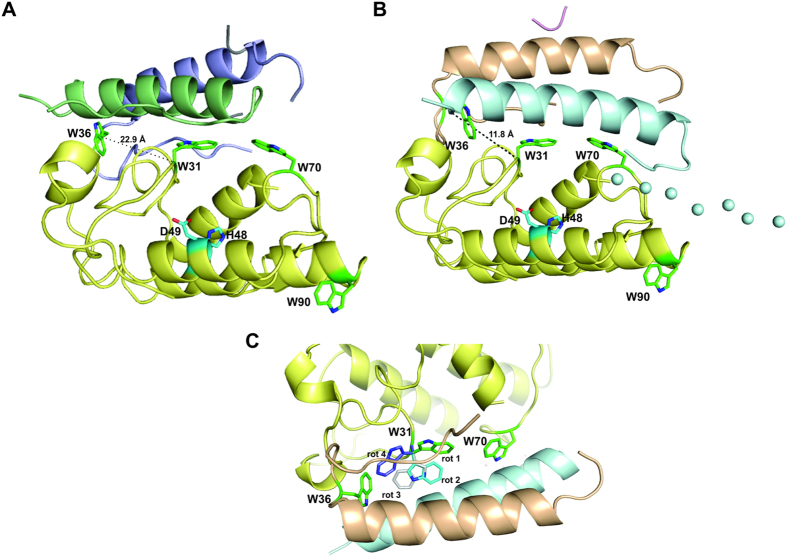
Figure 6. Structural studies of tryptophan residues at CA/CB interface in CTX heterodimer.
(A) Cartoon representation of crystal structure of CTX (PDB ID 3R0L). Chains α and β from CA are shown in blue and green, respectively. CB is shown in yellow. (B) Cartoon representation of SAXS model of CTX. Chains α, β, γ of CA are shown in wheat, blue, and purple, respectively. CB is shown in yellow. The loops of CA modeled as dummy atoms by CORAL software43 are shown as solid spheres. The tryptophan residues of CA (Trp36) and CB (Trp31, Trp70 and Trp90) are highlighted as green sticks. Residues of the catalytic site of CB (His48 and Asp49) are highlighted as cyan sticks. Cα distance between Trp36 of CA and Trp31 from CB is shown in black dashes. (C) A rotation of 180° of the cartoon representation of the SAXS model of CTX illustrated in panel B shows the possible rotamers of Trp31 from CB. Rotamer 1 represents the tryptophan rotamer presented by the CTX crystal structure, whereas other rotamers (2, 3 and 4) are obtained by the analysis of rotamer possibilities in the Coot software74. According to this software, rotamers 1, 2, 3 and 4 are present in 32%, 18%, 16% and 11% of the crystallographic structures available in the PDB.