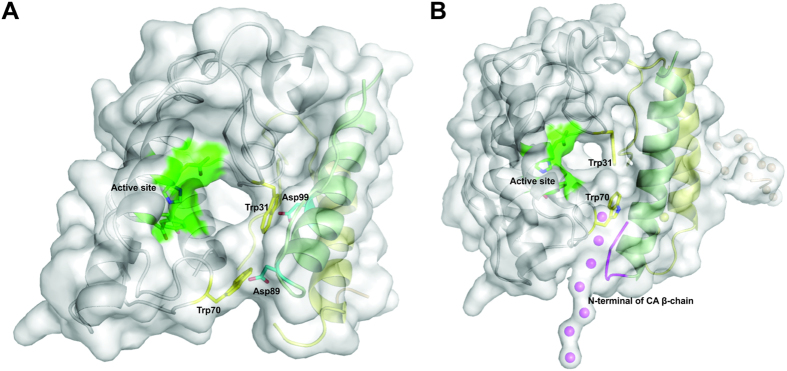
Figure 8. Accessibility of catalytic site from CB in CTX crystallographic and SAXS structural models.
(A) Cartoon representation of CTX crystal structure (PDB ID 3R0L) covered by white surface, where chains α, β, γ of CA are shown in green, yellow and wheat, respectively. CB is shown as a white cartoon. Trp31 and Trp70 residues from CBb isoform are highlighted by yellow sticks. The active site of CB (His48, Asp49, Tyr53 and Asp99) are represented in green. Asp89 and Asp99 residues of CA are shown as cyan sticks. CTX crystal structure is formed by a CA and a CB isoform from class I (CA2CBb isoforms). Despite partial blocking of the active site by Trp31 and Trp70 residues from the CBb isoform in a front view (residues that establish hydrogen bonds with Asp89 and Asp99 with β-chain of CA), it is possible to observe access to the catalytic site from a lateral view. (B) Cartoon representation of SAXS model covered by white surface where chains α, β, γ of CA are shown in green, yellow and wheat, respectively. CB is shown as a white cartoon. Trp31 and Trp70 residues from CB are highlighted by yellow sticks. Active site of CB (His48, Asp49, Tyr53 and Asp99) is represented in green. The disordered N-terminal region of β chain from CA (highlighted in magenta) is close to the accessible pocket of the catalytic site in this SAXS model. Since this region is very flexible, it can adopt different structural positions in crotoxin isoforms I and II, possibly contributing to catalytic site blocking in class I isoforms.