Abstract
Glucosylceramide (GlcCer) is the primary storage lipid in the lysosomes of Gaucher patients and a secondary one in Niemann-Pick disease types A, B, and C. The regulatory roles of lipids on the hydrolysis of membrane bound GlcCer by lysosomal β-glucocerebrosidase (GBA1) was probed using a detergent-free liposomal assay. The degradation rarely occurs at uncharged liposomal surfaces in the absence of saposin (Sap) C. However, anionic lipids stimulate GlcCer hydrolysis at low pH by up to 1,000-fold depending on the nature and position of the negative charges in their head groups while cationic lipids inhibit the degradation, thus showing the importance of electrostatic interactions between the polycationic GBA1 and the negatively charged vesicle surfaces at low pH. Ceramide, fatty acids, monoacylglycerol, and diacylglycerol also stimulate GlcCer hydrolysis while SM, sphingosine, and sphinganine play strong inhibitory roles, thereby explaining the secondary storage of GlcCer in Niemann-Pick diseases. Surprisingly, cholesterol stimulates GlcCer degradation in the presence of bis(monoacylglycero)phosphate (BMP). Sap C strongly stimulates GlcCer hydrolysis even in the absence of BMP and the regulatory roles of the intraendolysosomal lipids on its activity is discussed. Our data suggest that these strong modifiers of GlcCer hydrolysis affect the genotype-phenotype correlation in several cases of Gaucher patients independent of the types.
Keywords: Gaucher disease, cholesterol, fatty acids, sphingomyelin, acylglycerols, sphingosine, electrostatic interaction, anionic phospholipids, lysophospholipids, cationic lipids
Biological membranes are essentially composed of amphiphilic lipids and proteins that function primarily to maintain organelles in a specific pattern. To maintain the physiological turnover of cellular membranes and to enable autophagy, in case of cell starvation and hunger, internalized complex lipids, such as glycerolipids and sphingolipids, as well as proteins have to be degraded during the process of endosomal/lysosomal membrane digestion. This allows the final lipid degradation products to leave the compartment and reach the cytosol as nutrients for further synthesis and energy catabolism. While soluble macromolecules, such as proteins, glycoproteins, or oligosaccharides, can be degraded directly by soluble enzymes, the degradation of membrane lipids requires a much more complex disintegration and degradation system (1). These lipids play major roles in the cellular compartments and their accumulation in the endolysosomal compartments is associated with various storage disorders.
Gaucher disease remains the most common lysosomal storage disorder, which is mainly caused by the defective activity of lysosomal β-glucocerebrosidase (GBA1), thus resulting mainly in glucosylceramide (GlcCer) accumulation, particularly in the cells of the macrophage (2). GlcCer is formed on the cytosolic leaflet and is translocated to the luminal face of the Golgi, presumably by multidrug transporters (3), where it undergoes subsequent glycosidation reactions catalyzed by glycosyltransferases in the Golgi apparatus to form gangliosides and other glycosphingolipids. GlcCer, like other glycosphingolipids, could be transported to the plasma membrane through the vesicular protein secretory pathway, but an alternative pathway through the cytosol, probably by some glycolipid-transfer proteins, has been proposed earlier (4). After endocytosis, GlcCer and other glycosphingolipids of the plasma membrane reach the lysosomal compartments of the cell by a vesicular membrane flow, where they become degraded within the lumen of the acidic compartment of the cell as intraluminal vesicular components (5). Although it is known that GlcCer generated from lactosylceramide and other higher glycosphingolipids is degraded in the lysosome (6), it is not yet understood whether GlcCer reaching these compartments directly through endocytosis or autophagy is already degraded before reaching the lysosome. Saposin (Sap) C, one of the four homologous proteins (others being Saps A, B, and D) derived from the sequential cleavage of prosaposin (Sap precursor protein), is an essential cofactor that enhances the activity of GBA1 in GlcCer hydrolysis. Its deficiency causes an abnormal juvenile type of Gaucher disease characterized by GlcCer accumulation in the lysosomes (7, 8).
In addition to the GBA1 (also called GCase), a second distinct non-lysosomal β-glucocerebrosidase (GBA2) (9) with properties distinct from GBA1 in respect to inhibitor and artificial substrate specificities has been identified. It is a membrane-associated enzyme of the endoplasmic reticulum. Both GBA1 and GBA2 play active roles in the degradation of GlcCer, albeit in different cellular organelles, and deficiency in both leads to increased GlcCer storage in double knockout mice (10).
Anionic membrane lipids, such as phosphatidylglycerol (PG), phosphatidic acid (PA), phosphatidylinositol (PI), phosphatidylserine (PS), and bis(monoacylglycero)phosphate (BMP) as well as Sap C, a lysosomal lipid binding protein, have been identified to stimulate GlcCer degradation by GBA1 (11, 12). However, earlier experiments were conducted, at conditions highly dissimilar to what is currently reported, on the lipid compositions of the intraendosomal and intralysosomal compartments. Compared with earlier experiments that used 23 mol% cholesterol in a detergent-free liposomal assay mimicking the intralysosomal situation (11), recent studies indicate that the purified lysosomes contain only traces (as low as 5 mol%) of cholesterol (13, 14), much lower than the cholesterol content of the late endosomes. In the late endosomes, nondegradable cholesterol is sorted out mainly by two cholesterol transport proteins, Niemann-Pick disease protein types C1 and C2 (NPC1 and NPC2), as reported earlier (15–19). Of all the lipid catabolism stimulating anionic membrane lipids, BMP is the unique anionic phospholipid specifically found in lysosomes and late endosomes (6) that could reach up to 30–60 mol% of the total phospholipids in the lysosomal internal membranes (13, 20). BMP conveys negative surface charge to the luminal lysosomal vesicles, even at pH lower than 5 (21).
While previous experiments focused mainly on the stimulation of GlcCer degradation by anionic lipids, results on the inhibition of GlcCer degradation are only partly characterized (12). SM is known to inhibit cholesterol transport by NPC2 (18) and separate incorporation of SM and cholesterol, the two plasma membrane stabilizing lipids, into liposomes strongly inhibits GM2 activator protein-mediated GM2 hydrolysis (22), even in the presence of anionic lipids. Therefore, the inability of acid sphingomyelinase to degrade SM, as in Niemann-Pick disease types A and B, may not only lead to SM accumulation, but could also trigger secondary accumulation of other lipids, such as cholesterol, GM1, and GM2, among others (23). GlcCer is the most remarkable glycosphingolipid storage in almost all types of Niemann-Pick disease A, B and C (24, 25). The disparity regarding the use of 23 mol% cholesterol in the liposomal preparations of a previously developed assay to measure GlcCer degradation by GBA1 (11), compared with low (about 5 mol%) cholesterol reported in recent studies (13, 14), leads to the modifications of the existing assay for the same purpose with necessary optimizations.
In this study, a modified detergent-free liposomal assay to measure GlcCer degradation was used to examine the roles of anionic and cationic lipids on GlcCer degradation via electrostatic interactions, as well as the roles played by SM, ceramide (Cer), cholesterol, and the lysosomal lipid degradation products such as lyso-lipids, diacylglycerol (DAG), monoacylglycerol (MAG), sphingoid bases, and fatty acids. The aim is to mimic the in vivo conditions of intralysosomal compartments.
MATERIALS AND METHODS
Materials
Dioleoyl-l-α-phosphatidylcholine (DOPC), BMP, sulfatide, 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine (EPC), N1-[2-((1S)-1-[(3-amino propyl)amino]4-[di(3-amino-propyl)amino]butylcarboxamido)ethyl]3,4-di[oleyloxy] benzamide [multivalent cationic lipid (MVL5)], 1,2-di-O-octadecenyl-3-trimethylammonium propane (DOTMA), and C18:1-sphingosine were obtained from Avanti Polar Lipids (Alabaster, AL).
Cholesterol, dipalmitoyl-PA, dipalmitoyl-PS, distearoyl-PG, PI, 1,2-dipalmitoylglycerol, 1-stearoylglycerol, C16:0-lysophosphatidylcholine (lyso-PC), stearic acid, C18:1-Cer, C18:1-sphinganine, dihexadecyl phosphate (DHP) (also called dicetyl phosphate), and 4-methylumbelliferyl-β-D-glucopyranoside (MUG) were purchased from Sigma (Taufkirchen, Germany). C18:1-SM was obtained from Matreya (Pleasant Gap, PA). GM1 and GM3 were from Fidia Research Laboratories [Abano Terme (PD), Italy] and GM2 was available in our laboratory.
Synthesis of radiocarbon-labeled GlcCer, [14C]GlcCer
GlcCer labeled in its fatty acid residue was synthesized by acylating glucosylsphingosine using [1-14C]stearic acid N-succinimidyl ester with a specific radioactivity of 2.14 TGb/mol, as described previously (26). Glucosylsphingosine was synthesized from appropriately activated glucose and a protected sphingosine following published procedures (27, 28) with modifications in the glycosylation protocol (29). The purified [14C]GlcCer was characterized by ESI-QTOF-MS.
GBA1 source
The modified GBA1 (Cerezyme®) manufactured by Genzyme (Cambridge, MA) by recombinant DNA technology using mammalian cell culture (Chinese hamster ovary) was a remnant of the enzyme used in our previous study (11). The enzyme was kept in 20 mM sodium citrate buffer containing 150 mM sodium chloride (pH 4.5, except as otherwise stated). The specific activity of GBA1, as measured with MUG assay (30), was 0.083 μmol MUG/min/mg enzyme.
Isolation and purification of Sap C
Sap C was isolated from the spleen of a Gaucher disease patient, as described previously (31). The purity of the Sap C preparation was ascertained by polyacrylamide gel electrophoresis with silver staining, Western blotting, and sequencing.
Preparation of liposomes
Liposomes were prepared as previously described (21, 32). Neutral liposomes contained 5 mol% cholesterol, 94 mol% DOPC, and 1 mol% [14C]GlcCer (as a radiolabeled substrate). Negatively or positively charged liposomes generally consisted of 5 mol% cholesterol, 74 mol% DOPC, 1 mol% [14C]GlcCer, and 20 mol% anionic lipid (BMP, PA, PG, PI, PS, DHP, sulfatide, GM1, GM2, or GM3) or cationic lipid (EPC, MVL5, DOTMA) unless otherwise stated. The appropriate amount of DOPC was adjusted to allow for the variation of the compositions of liposomes.
Liposomes were prepared by mixing appropriate amounts of lipids from stock solutions and the solvents from the resulting solution mixtures were evaporated under a stream of nitrogen. The lipid mixture was hydrated in 20 mM citrate buffer (pH 4.5, except as otherwise stated) containing 150 mM NaCl (except as otherwise stated) to a final lipid concentration of 200 nmol/ml. The dispersion was vortexed, sonicated in a Branson sonifier (Danbury, CT) at 120 W for 30 s, and subjected to eight freeze-thaw cycles. Large unilamellar vesicles were prepared by extrusion through polycarbonate filters with a pore size of 100 nm mounted in tandem in a mini-extruder (LiposoFast; Avestin, Ottawa, Canada). Samples were subjected to 21 passes.
Liposomal GlcCer assay
The liposomal assay mixture for determination of GBA1 activity contained 40 μl (8 nmol total lipid) of a liposomal preparation and 60 ng (except as otherwise stated) of GBA1 and the volume was adjusted by the addition of buffer (20 mM sodium citrate buffer containing 150 mM sodium chloride, pH 4.5, except as otherwise stated) to a final volume of 100 μl. After 30 min of incubation at 37°C, the reaction was stopped by the addition of 280 μl chloroform/methanol 2/5 (v/v) to obtain a homogeneous phase. Lipids were dried under a stream of nitrogen and redissolved with 60 μl chloroform/methanol 1/1 (v/v), vortexed, and centrifuged. The lipids of the extract were separated sequentially with chloroform/methanol 4/1 (v/v) by thin-layer chromatography using a high-performance thin-layer chromatography plate (Merck, Darmstadt, Germany). Radioactive bands were visualized with a Typhoon FLA 7000 phosphoimager (GE Healthcare, Buckinghamshire, UK) and the quantification was performed with the image analysis software “ImageQuant™ TL” from the same manufacturer.
RESULTS
Despite the key roles played by GBA1 in the degradation of GlcCer in the intralysosomal system, its stimulation and inhibition studies were only partially characterized. Earlier experiments remain defective, as they were conducted at conditions highly dissimilar to those from current reports on the lipid compositions of the intralysosomal vesicles in recent studies. Furthermore, the regulatory effects of important lipids, such as SM and cholesterol, which turned out to be a strong inhibitor and a stimulator of GBA1 activity, respectively, had not been probed previously.
As reported earlier, the lipid compositions of the intraendosomal and intralysosomal vesicles change drastically due to lipids sorting during endocytosis. Cholesterol is sorted out by a mechanism involving mainly two cholesterol transfer proteins, NPC2 and NPC1, with the former being a soluble intralysosomal glycoprotein that transfers cholesterol to the latter, which is a transmembrane protein in the endosomal compartment (17). Hence, the resulting cholesterol content in the intralysosomal vesicles tends to be much lower than that in the intraendosomal vesicles. Recent studies indicate that the purified lysosomes contain only traces (as low as 5 mol%) of cholesterol (13, 14) compared with 23 mol% cholesterol used in the liposomal preparations in a previously developed assay to measure GlcCer degradation by GBA1 (11), thereby leading to the modifications of the existing assay for the same purpose with necessary optimizations.
GlcCer degradation assay optimization
The influences of the enzyme concentrations, pH, incubation time, and the ionic strength on the degradation of the liposomal GlcCer by GBA1 were studied using an optimized detergent-free liposomal assay.
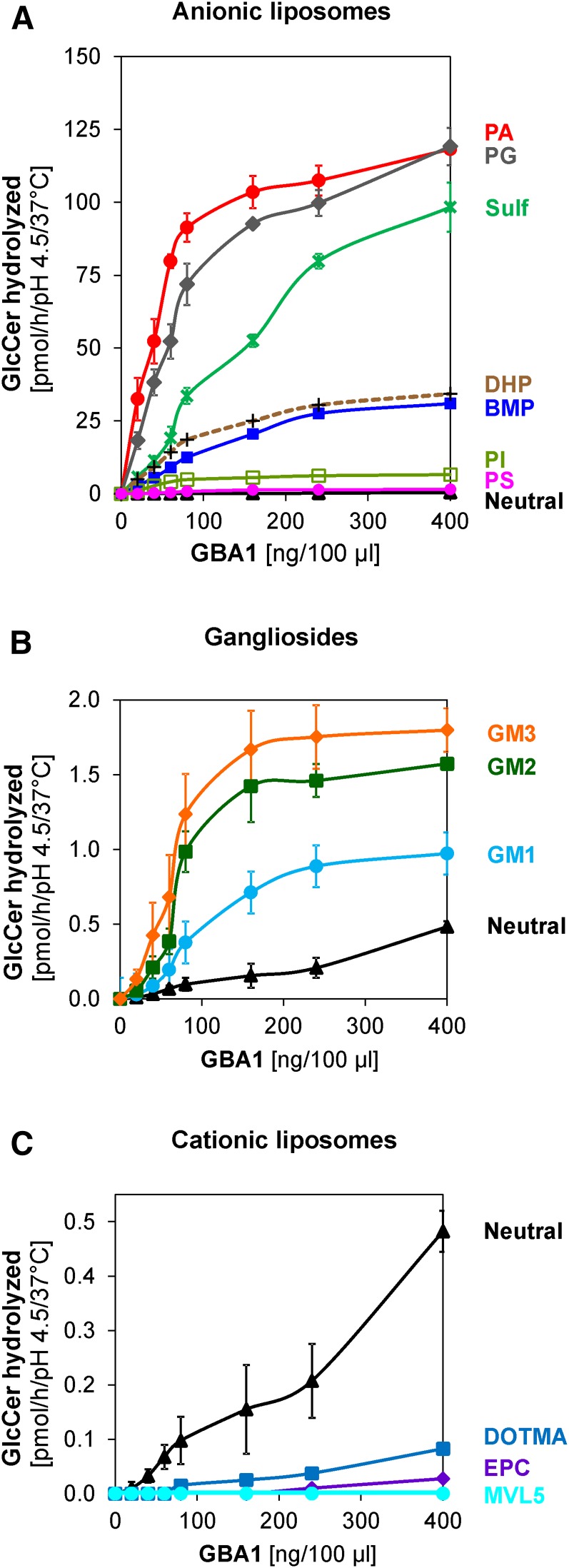
Figure 1A shows the dependence of GlcCer degradation on GBA1 concentration using neutral and anionic lipid-containing liposomes. In a neutral liposome, which is composed of 5 mol% cholesterol and 1 mol% radiolabeled GlcCer with DOPC being the host lipid, the degradation of GlcCer is extremely low under all the enzyme concentrations used, with the observed values ranging between 0.01 and 0.5 pmol/h when the enzyme concentration was increased from 20 to 400 ng per assay volume. In anionic lipid-containing liposomes prepared by the addition of 20 mol% BMP to the lipid mixture used for the neutral liposomes (and DOPC contents adjusted), the turnovers increased from 3 to 31 pmol/h within the same enzyme concentration range (20 to 400 ng per assay volume). The replacement of BMP with PA gives rise to extremely high turnovers ranging from 33 pmol/h at 20 ng GBA1 per assay up to 118 pmol/h at 400 ng GBA1 per assay. In the presence of anionic lipids, the linearity of the dependence of the GlcCer degradation on the enzyme concentration is maintained up to 100 ng GBA1 per assay, after which the degradation levels off gradually. Therefore, the enzyme concentration for further works was limited to 60 ng GBA1 per assay volume. At 60 ng GBA1 per assay volume, BMP and PA greatly stimulate GlcCer up to 129- and 1,140-fold, respectively, compared with the low turnover of 0.07 pmol/h observed in the neutral liposome at the reported enzyme concentration. In the presence of 20 mol% PA, GlcCer degradation occurs rapidly and linearly until about 50% of the total GlcCer is degraded at low enzyme concentration, but levels off at about 60% degradation at high GBA1 concentration correlating with the degradation of all the GlcCer available in the outer leaflet of the membrane.
Fig. 1.
Assay optimization for the hydrolysis of membrane bound GlcCer by GBA1. Neutral liposomes contain 5 mol% cholesterol, 1 mol% [14C]GlcCer, and 94 mol% DOPC. Anionic liposomes contain 20 mol% anionic lipid (BMP or PA), 5 mol% cholesterol, 1 mol% [14C]GlcCer, and 74 mol% DOPC. The enzyme concentration is 60 or 400 ng GBA1/100 μl assay in anionic and neutral liposomes, respectively, and the experiments were carried out at pH 4.5 under 30 min incubation and at 37°C (except as otherwise stated). GlcCer hydrolysis is enzyme dose dependent (A) and time dependent (B) in neutral (black), BMP-containing (blue), and PA-containing liposomes (red). GBA1 activity is pH dependent in PA-containing (C) and BMP-containing (D) liposomes. High ionic strength inhibits the hydrolysis of GlcCer by GBA1 even in the presence 20 mol% PA (E) or 20 mol% BMP (F), using 20 mM (red) and 50 mM (blue) citrate buffers (pH 4.5) containing 0–300 mM NaCl. Mean ± SEM (n = 4–6).
In the time course studies for the GlcCer degradation by GBA1 (Fig. 1B), significant amounts of GlcCer degradation have already been observed at short incubation time in the BMP-containing liposomes, with the optimum achieved in 30 min at pH 4.5 using 60 ng GBA1 per assay volume. The linearity is maintained up to 30 min incubation time and the value levels off at about 10% degradation of the GlcCer residing in the outer leaflet of the membrane. However, a much more rapid GlcCer turnover rate was observed in PA-containing liposomes, even at short incubation time up to 30 min at which it tends to occur linearly until it levels up at about 50% of the total GlcCer showing the complete degradation of the total GlcCer residing in the outer leaflet of the membrane.
The maturation from endosomes to lysosomes and the formation of intraluminal membranes at the stage of late endosomes, the multivesicular body, requires an acidic environment (33). Low pH values of endosomes (pH 5–6) and lysosomes (pH 4.2–5) are a characteristic feature of the acidic compartments (33) and this low pH is generated by the action of V-type ATPases and serves various functions (34). Accordingly, almost all lysosomal enzymes and lipid binding proteins require low pH for their optimal activities. As observed from Fig. 1C, the pH profile of the GlcCer degradation by GBA1 in PA-containing liposomes gives broad peaks at 5–20 mol% PA, thus giving the pH optima between 4.0 and 5.5. The peaks become broader as the concentration of PA is increased from 20 to 40 mol%, with an appreciable amount of GlcCer degradation occurring even at higher pH and at high PA concentrations in the liposomes. A similar broad peak was also observed with neutral liposomes (data not shown). However, in the presence of high BMP concentrations (>20 mol%), the peak becomes much narrower with pH optimum observed at 4.5, showing the sensitivity of the enzyme to high BMP concentration (Fig. 1D). It is worthy to note that a much higher turnover was recorded from 153 to 883 nmol/h/mg GBA1 when BMP concentration was increased from 20 to 25 mol%, respectively, and further increments to 1,475 and 1,573 nmol/h/mg GBA1 were recorded with 30 and 40 mol% BMP, respectively (Fig. 1D).
In a previous study that investigated the functions of isolated lysosomes by measuring mucopolysaccharide degradation, the best lysosome stability was recorded at low ionic strength (35). Moreover, high ionic strength was observed to inhibit the activities of lysosomal enzymes, such as lysosomal phospholipase A2 (36), as well as lysosomal lipid binding proteins, such GM2 activator protein (22). The inhibitory effects of high ionic strength on the GBA1 action on GlcCer in anionic liposomes containing 20 mol% PA or 20 mol% BMP are shown in Fig. 1E, F, respectively using 20 or 50 mM citrate buffer (pH 4.5) containing 0–300 mM NaCl. The optimum GlcCer degradation was observed using 20 mM citrate buffer containing 0–150 mM NaCl, while a high decrease was observed at NaCl concentrations greater than 150 mM. Reductions in GBA1 activity of about 2% and 13% were observed when the concentration of the citrate buffer was changed from 20 to 50 mM in the absence of NaCl in PA- and BMP-containing liposomes, respectively. Also, the addition of 300 mM NaCl to the 20 mM citrate buffer solution led to drastic reductions of about 26% and 86% of GBA1 activity in PA- and BMP-containing liposomes, respectively. Therefore, the use of NaCl in further assays was limited to 150 mM.
Anionic lipids stimulate GlcCer degradation, but cationic lipids play inhibitory roles
The influence of several phospholipids, gangliosides, and sulfatide, all carrying negatively charged head groups, was investigated. PG, PA, PI, and PS are found in the cytosolic leaflets of cellular membranes while BMP is generated in the luminal vesicles of the late endosomes and lysosomes. Synthetic anionic lipid (DHP) and synthetic cationic lipids (DOTMA, EPC and MVL5) were also used in the experiment so as to measure the interactions of various lipids with different charges on the GlcCer degradation by GBA1. GlcCer degradation, which occurs rapidly and in a linear fashion at enzyme concentrations less than 100 ng GBA1 per assay volume, levels off thereafter and, henceforth, 60 ng GBA1 per assay volume was set as the standard enzyme concentration condition for the comparison in all liposome types. In neutral liposomes containing neither anionic nor cationic lipids (Fig. 2A, C), the GlcCer degradation reached was extremely low (0.07 ± 0.02 pmol/h) under standard condition. The GlcCer degradation was strongly stimulated by anionic phospholipids derived from the plasma membrane (PA and PG) as well as sulfatide, in the order: PA (1,140-fold) > PG (746-fold) > sulfatide (273-fold) (Fig. 2A). However, moderate stimulatory roles were displayed by DHP, BMP, and PI, but with PS only displaying a weak stimulatory role (Fig. 2A), in the order: DHP (203-fold) > BMP (129-fold) > PI (70-fold) > PS (7-fold). Relatively weak stimulations were observed with the negatively charged gangliosides, in the order: GM3 (10-fold) > GM2 (6-fold) > GM1 (3-fold) (Fig. 2B).
Fig. 2.
Regulatory roles of anionic and cationic membrane lipids on the hydrolysis of membrane bound GlcCer by GBA1. A: GlcCer digestion is strongly stimulated by PA (red), PG (gray), and sulfatide (green) and moderately stimulated by DHP (brown), BMP (blue), and PI (light green), but only weakly stimulated by PS (pink). B: Only weak stimulations are observed with gangliosides; GM1 (light blue), GM2 (green), and GM3 (orange) as well as in neutral (black) liposomes, while cationic lipids, such as EPC (purple), DOTMA (blue), and MVL5 (light blue), inhibit the GlcCer hydrolysis (C). Mean ± SEM (n = 4–6).
Contrary to the stimulatory effect of anionic lipids, cationic lipids exhibit inhibitory roles on the GlcCer degradation by GBA1 (Fig. 2C). Even at a higher GBA1 concentration (400 ng/ assay volume), MVL5 completely inhibits the GlcCer degradation, while the lowest inhibitory role was exhibited by DOTMA. Different inhibitory roles played by the cationic lipids might be a result of the positions of the positive charges in each of the molecules. DOTMA, which has the least inhibitory roles, has its positive charge more localized in the lipophilic part of the membrane away from the interface where the enzyme acts on the substrate, while MVL5, with the strongest inhibitory role, has its five positive charges on its head group at low pH values close to the degradation site. Therefore, it is apparent that electrostatic interaction plays very important roles in the degradation of GlcCer in the lysosomal membranes, especially in the association of different anionic membranes with the positively charged GBA1 at low pH.
GlcCer degradation increases with increase in anionic lipid concentration
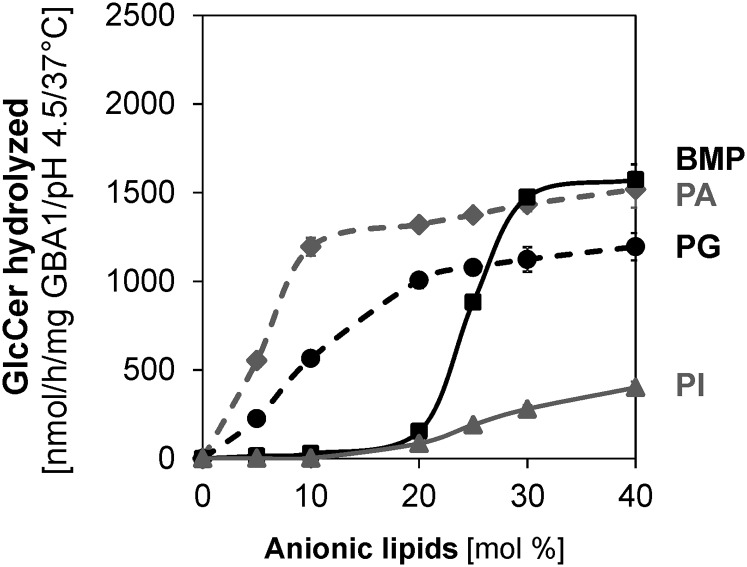
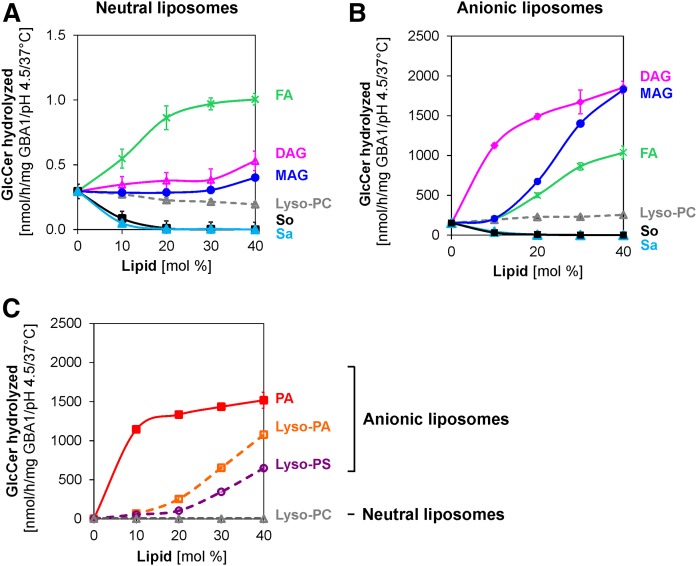
As reported earlier in this work (Fig. 2A), anionic phospholipids, such as PA, PG, BMP, and PI, stimulate the GlcCer degradation in different capacities, with PA and PG being very strong GlcCer degradation stimulators, the former stronger than the latter, while BMP and PI are moderate stimulators. Therefore, it is pertinent to investigate how the variations in the concentrations of these anionic phospholipids influence GlcCer degradation. As shown in Fig. 3, 5 mol% of PA and PG significantly stimulate the degradation of GlcCer with turnovers of 553 and 226 nmol/h/mg GBA1, respectively, under standard experimental conditions and as the concentrations of PA and PG increase from 5 to 30 mol%, GlcCer degradation increases by 3- and 5-fold, respectively. With BMP, a significant level of GlcCer degradation (15 nmol/h/mg GBA1) was only observed when its concentration in the liposome was about 20 mol% and a much lower degradation (2 nmol/h/mg GBA1) was recorded with PI under the same conditions as BMP. As the concentration of the anionic lipids increase, the GlcCer degradation levels off at about 50% in the PA- and BMP-containing liposomes, indicating the total degradation of GlcCer residing in the outer leaflets of the liposomes, while a slow degradation rate was observed with PI even at 40 mol%. The high GlcCer degradation rate stimulated by PA and PG may not be of relative physiological relevance, as both lipids are degraded at the low pH of the lysosomes by lysosomal phospholipases, but BMP has been reported to be enriched in the intravesicular membranes of the late endosomes and lysosomes with a significant amount of PI (13, 20).
Fig. 3.
Anionic lipids stimulate membrane bound GlcCer hydrolysis by GBA1 in a concentration-dependent manner. Liposomes containing 0–40 mol% anionic lipids (PI, PG, PA, or BMP) were used. The experiments were carried out using 60 ng GBA1 per assay under 30 min incubation and at 37°C. Mean ± SEM (n = 4–6).
More importantly, BMP, which is a unique anionic lysophospholipid found in the intravesicular membranes of the late endosomes and lysosomes, is highly stable at the lysosomal pH and accounted for up to 70% of total phospholipids of the intralysosomal membranes (20). Therefore, the dependence of the GlcCer hydrolysis on lysosomal BMP concentration would be of great physiological importance. As observed with the experiments using 0 to 40 mol% BMP, a great jump in the GlcCer degradation (about 6-fold) was recorded when the liposomal BMP concentration increased from 20 to 25 mol% and this trend continued until a maximum degradation was attained at 30 mol% under the standard assay conditions (Fig. 3). The sigmoidal curve obtained with BMP could be of high significance to show high affinity of GBA1 for BMP, as the accumulation of GlcCer in the lysosome can still be recorded when BMP is present, but inadequate to stimulate the enzymatic reaction satisfactorily. A similar GlcCer degradation jump, albeit to a lesser extent (2-fold), was also observed with PI.
Cholesterol and Cer stimulate GlcCer degradation, while SM plays a strong inhibitory role
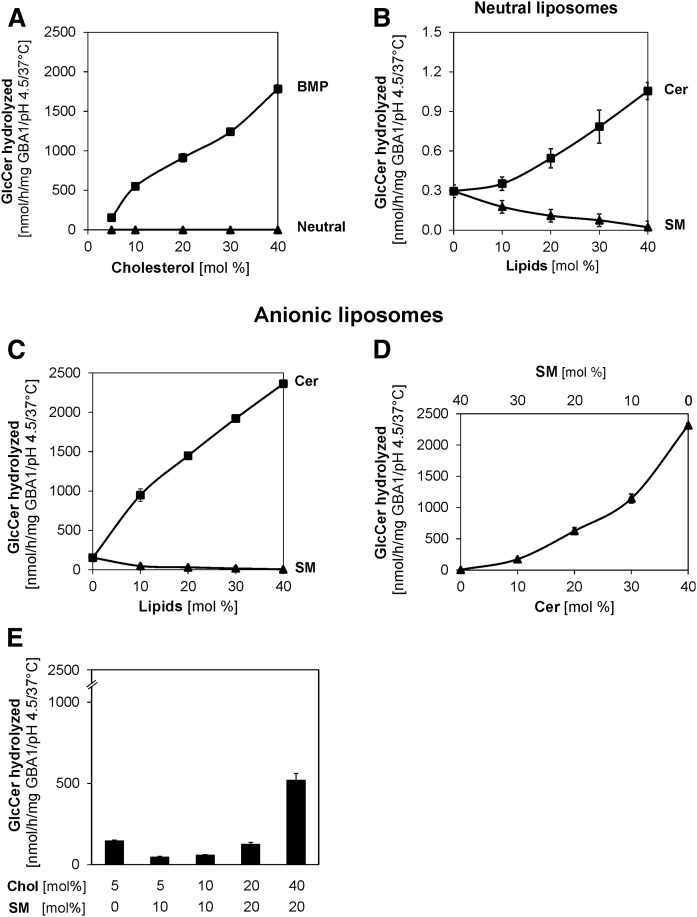
Although the cholesterol content of the lysosome is low, as reported earlier (13, 14), deficiencies in one or both of its two main transport proteins, NPC1 and NPC2, in the late endosome leads to its accumulation in the endolysosomal compartments, thus resulting in Niemann-Pick disease type C. In Niemann-Pick disease type C, secondary accumulations of a number of glycolipids, such as GM1, GM2, GlcCer, and lactosylceramide, have been reported (24, 25, 37). Previous works in our laboratory showed that an increased level of membrane-stabilizing cholesterol inhibits the activities of lysosomal sphingolipid activator proteins, such as GM2 activator protein (22), Sap A (38), and Sap B (39), but has no significant effect on SM hydrolysis by acid sphingomyelinase (21). Therefore, its effect on GlcCer degradation was studied in this work using both neutral and BMP-containing liposomes (Fig. 4A). In the presence of BMP, doubling the liposomal cholesterol content from 5 to 10 mol% increases the GlcCer turnover from 153 to 550 nmol/h/mg GBA1. This 4-fold stimulation is contrary to the inhibitory roles of cholesterol in the GM2 activator protein-induced and hexosaminidase A-catalyzed GM2 hydrolysis (22). As the concentration of cholesterol increases, GlcCer degradation by GBA1 also increases, reaching 12-fold stimulation at 40 mol% cholesterol. However, in the neutral liposome (devoid of anionic lipids), the effect of cholesterol on GlcCer degradation is insignificant (Fig. 4A).
Fig. 4.
Cholesterol and Cer strongly stimulate membrane bound GlcCer hydrolysis by GBA1, while SM plays a strong inhibitory role. Cholesterol strongly stimulates GlcCer hydrolysis in the presence of BMP (A). Liposomes contain 5–40 mol% cholesterol, 1 mol% [14C]GlcCer (Neutral), and, in addition, 20 mol% BMP. Cer stimulates GlcCer hydrolysis, while SM displays an inhibitory role in the neutral liposomes (B) and in the presence of BMP (C). Liposomes contain 5 mol% cholesterol, 1 mol% [14C]GlcCer (Neutral), and, in addition, 20 mol% BMP with various concentrations of Cer and SM. GlcCer hydrolysis is strongly stimulated as SM is gradually replaced with Cer (D) in the presence of BMP with the total SM and Cer contents being 40 mol% at any time. Cholesterol plays stimulatory roles even in the presence of SM (E) in BMP-containing liposomes. The experiments were carried out using 10,000 and 60 ng GBA1 per assay for neutral and BMP-containing liposomes, respectively, at pH 4.5, under 30 min incubation and at 37°C. Mean ± SEM (n = 4–6).
In Niemann-Pick disease types A and B resulting from the primary accumulation of SM, secondary accumulations of other lipids, such as cholesterol, GM1, and GM2, among others, have been reported (23). SM has also been shown to inhibit cholesterol transfer by NPC2 (18) as well as GM2 activator protein-induced and hexosaminidase A-catalyzed GM2 hydrolysis (22), but is degraded to Cer by ASM in the late endosome (40).
In neutral liposomes, SM exhibits strong inhibition to GlcCer degradation, while a stimulatory role is displayed by Cer (Fig. 4B), and these inhibitory and stimulating roles played by SM and Cer, respectively, become much stronger in the presence of BMP (Fig. 4C). With BMP, only 10 mol% SM inhibits GlcCer degradation to a very great extent (from 153 to 47 nmol/h/mg GBA1), representing 69% inhibition, and the inhibition becomes stronger as SM contents increase up to 40 mol%. On the other hand, 10 mol% Cer displayed 6-fold stimulation in the presence of BMP and up to 15-fold stimulation was observed as the Cer contents were increased to 40 mol%. To mimic the effect of SM hydrolysis by the acid sphingomyelinase, we replaced the SM contents (from 40 to 0 mol%) correspondingly with Cer (from 0 to 40 mol%) (Fig. 4D). The observed strong stimulation of GlcCer degradation is physiologically important, as SM degradation to Cer by acid sphingomyelinase could enhance GlcCer hydrolysis because the accumulation of the SM in cells leads to secondary accumulation of GlcCer, as observed in Niemann-Pick disease types A, B, and C (25, 42). At > 20 mol% Cer, the GlcCer degraded exceeded 60%, indicating that Cer enabled the GBA1 to have access not only to the GlcCer molecules on the outer leaflet, but also to those residing in the inner leaflet.
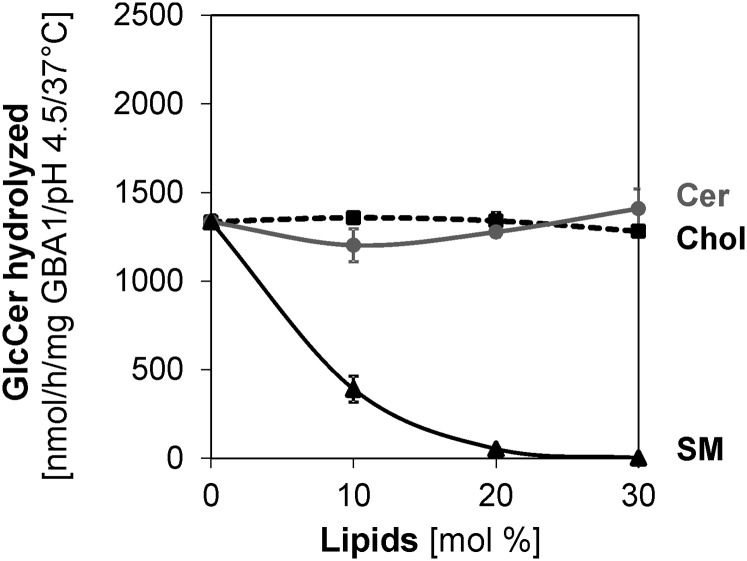
As observed above, cholesterol stimulates, while SM inhibits, GlcCer degradation. It is hence desirable to know the combined effects of SM and cholesterol on GlcCer degradation, as cholesterol has been reported to have higher affinity for SM-rich membranes than for any other membrane types (42). As observed in Fig. 4E, cholesterol still stimulates GlcCer degradation, albeit to a lesser extent, when associated with SM, while the inhibitory roles of SM were ameliorated by cholesterol, especially when present at high concentrations. In contrast, when the anionic lipid was changed from BMP to PA, Cer and cholesterol played no significant roles, while the strong inhibitory role played by SM was retained (Fig. 5).
Fig. 5.
In the presence of PA as the anionic lipid, cholesterol (Chol), and Cer regulatory effects on membrane bound GlcCer hydrolysis by GBA1 are insignificant, while SM plays a strong inhibitory role. Liposomes contain 5 mol% cholesterol, 1 mol% [14C]GlcCer, 20 mol% PA, and 0–30 mol% cholesterol, Cer, or SM (as indicated) with DOPC being the host lipid. The experiments were carried out using 60 ng GBA1 per assay under 30 min incubation and at 37°C. Mean ± SEM (n = 4–6).
Various lysosomal lipid degradation products influence GlcCer degradation differently
In the lysosomes, phospholipids and glycolipids are degraded by lysosomal enzymes leading to other lipid products, such as lyso-PC, DAG, MAG, fatty acids, sphingosine, and sphinganine. Therefore, the effects of these intermediates and final lipid degradation products on GlcCer degradation were examined. In neutral liposomes, lyso-PC shows no significant effect on GlcCer degradation, except at high concentration (Fig. 6A), whereas DAG and MAG exhibit slight stimulating roles. Stearic acid stimulates GlcCer degradation more than 2-fold at 10 mol% fatty acid, and this increases as the fatty acid contents increase, with its stimulatory effect much higher than those obtained with DAG and MAG. The stimulatory effect of stearic acid could probably be due to its negative charges, which could interact at the liposomal surface with the protonated enzyme protein electrostatically at the low pH. Sphingosine and sphinganine (10 mol% each), which both carry positive charges at low pH, inhibit the degradation of GlcCer very strongly by up to about 80%. In the presence of BMP (Fig. 6B), DAG has the strongest stimulatory effect on GlcCer turnover with a 7.4-fold increase observed at just 10 mol%. MAG and stearic acid also play significant stimulatory roles at higher concentrations, while similarly strong inhibitory roles are displayed by sphingosine and sphinganine compared with the observations with neutral liposomes. Lyso-PC plays no significant role on GlcCer hydrolysis, despite the presence of BMP. This may be related to the neutral charge on its head group, as experiments with other lyso-lipids carrying negative charges on the head groups (lyso-PA and lyso-PS) show that both lyso-PA and lyso-PS strongly stimulate the degradation of GlcCer in the absence of BMP, albeit to a much lesser extent than PA (Fig. 6C).
Fig. 6.
Stimulatory and inhibitory roles of various lysosomal lipid degradation products on membrane bound GlcCer hydrolysis by GBA1. Stearic acid, FA (green), DAG (pink) and MAG (blue) stimulate GlcCer hydrolysis; sphingosine (So) (black) and sphinganine (Sa) (light blue) strongly inhibit GlcCer hydrolysis, while lyso-PC (gray) plays different roles in neutral liposomes (A) and in the presence of BMP (B). In the absence of BMP (neutral liposomes), lyso-phospholipids carrying negatively charged head groups [lyso-PA (orange) and lyso-PS (purple), as indicated] strongly stimulate GlcCer hydrolysis more highly than the neutral lyso-PC, but to a lesser extent compared with PA (red) (C). The experiments were carried out using 10,000 and 60 ng GBA1/100 μl assay for neutral and anionic liposomes, respectively, at pH 4.5, under 30 min incubation and at 37°C. Mean ± SEM (n = 4–6).
Sap C induces GlcCer degradation even in the absence of anionic phospholipids
The inherited defects in Sap C, one of the sphingolipid activator proteins, cause a lethal GlcCer accumulation in a juvenile form of Gaucher disease. GBA1 activity is enhanced by Sap C in the lysosomes (43, 44) and a series of in vitro studies confirm the interactions between this enzyme and Sap C (45–47). While the in vitro hydrolysis of the membrane-bound complex glycosphingolipids, such as GM1 (48) and GM2 (22), by their respective hydrolases hardly takes place in the absence of both sphingolipid activator proteins and anionic lipids, the smaller GlcCer is already degraded in vitro by GBA1 in the presence of anionic lipids, even in the absence of Sap C (11). The non-requirement of Sap C for the degradation of GlcCer, once BMP is present, is also confirmed in this in vitro study, although lower liposomal cholesterol contents were used compared with its contents in the previous work (11).
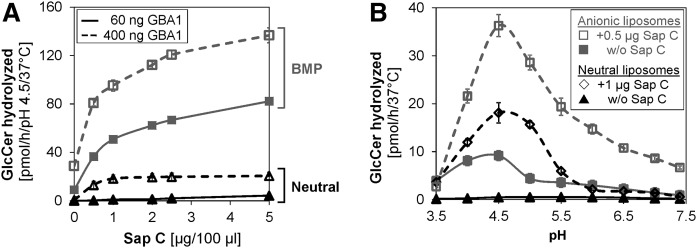
GlcCer degradation is Sap C dose dependent (Fig. 7A). In neutral liposomes, negligible GlcCer degradation was recorded at the low GBA1 concentration (60 ng per assay volume) even at high Sap C concentrations (up to 5 μg). However, at about a 6.7-fold increase in the enzyme concentration (400 ng GBA1 per assay volume) and in the absence of BMP, a 52-fold stimulation was recorded at 0.5 μg Sap C. More interestingly, 0.5 μg Sap C increases the GlcCer turnover by 148- and 324-fold at low and higher GBA1 concentration, respectively, in the presence of BMP. This shows that BMP could greatly facilitate GlcCer degradation, even at low GBA1 concentration and in the absence of Sap C, while higher Sap C concentration could adequately stimulate GlcCer degradation in the absence of anionic lipids once the GBA1 concentration is adequately high. The Sap C-induced GlcCer degradation is pH dependent (Fig. 7B), with the pH optimum obtained at 4.5 and the sharpest peak reached when both Sap C and BMP are present at low enzyme concentration, suggesting that the presence of both BMP and Sap C are required for optimal GlcCer degradation, although the absence of one could be compensated for by the presence of the other in the appropriate proportions.
Fig. 7.
Sap C facilitates the hydrolysis of membrane bound GlcCer by GBA1 even in the absence of anionic phospholipid (BMP). GlcCer hydrolysis is Sap C dose dependent (A) and the Sap C-induced GlcCer hydrolysis by GBA1 is significant even in the absence of BMP, but at higher GBA1 concentration. However, the presence of BMP tremendously enhances the Sap C activity. The pH profile of GlcCer hydrolysis becomes narrower in the presence of both Sap C and anionic lipid, BMP (B). The pH optimum for all experiments remains 4.5 in the presence or absence of Sap C, with the narrowest pH profile obtained during the combined presence of both Sap C and BMP. The experiments were carried out using 400 and 60 ng GBA1 per assay for neutral and anionic liposomes (containing 20 mol% BMP), respectively, at pH 4.5, under 30 min incubation and at 37°C. Mean – SEM (n = 4–6).
Cholesterol and Cer strongly stimulate Sap C activity while SM plays a strong inhibitory role
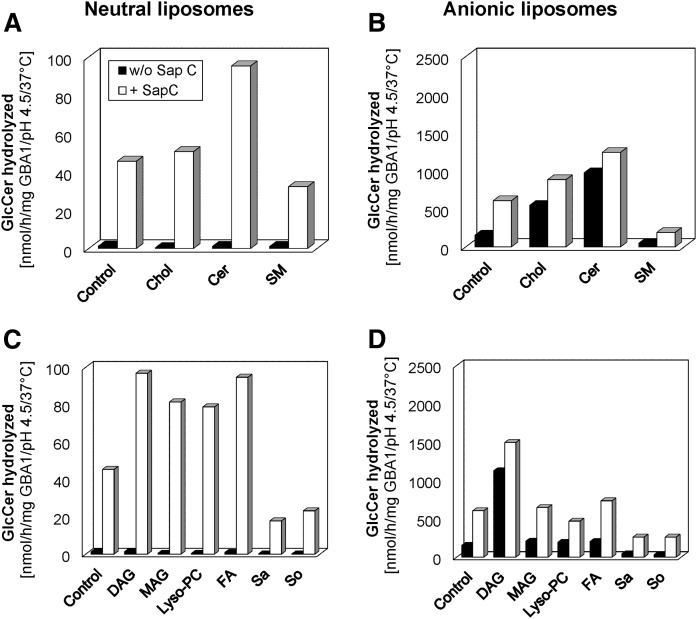
As evident in Fig. 8A, GlcCer degradation, which hardly takes place in the absence of BMP and in neutral liposomes without Sap C, was greatly stimulated up to 73-fold by 1 μg Sap C at 400 ng GBA1 per assay volume, even in the absence of BMP. The Sap C activity was enhanced slightly by cholesterol and strongly by Cer by up to 1.1- and 2-fold, respectively, while SM inhibited its activity by more than 37%. GlcCer degradation, which had already been stimulated by BMP (up to 129-fold, compare control in Fig. 8A with control in Fig. 8B) at low GBA1 concentration, was enhanced an additional 3.3-fold by 0.5 μg Sap C (Fig. 8B, control). In the presence of BMP, both cholesterol and Cer strongly enhanced Sap C activity by 1.5- and 2-fold, respectively. When BMP was present, Sap C slowly ameliorated the strong inhibition of GlcCer degradation exhibited by SM by 3-fold compared with the 36-fold Sap C-induced stimulation observed when 10 mol% SM was present in the liposomes in the absence of BMP.
Fig. 8.
Stimulatory and inhibitory roles of various lysosomal lipid degradation products on Sap C-induced hydrolysis of membrane bound GlcCer by GBA1. GlcCer hydrolysis is insignificant in the absence of both Sap C and BMP. Sap C-induced GlcCer hydrolysis by GBA1 is enhanced strongly by Cer and slightly by cholesterol (Chol), but inhibited by SM in neutral liposomes (A) and in the presence of BMP (B), respectively. Also, the Sap C-induced GlcCer hydrolysis by GBA1 is strongly enhanced by DAG, MAG, stearic acid, and lyso-PC, but strongly inhibited by sphingosine (So) and sphinganine (Sa) in neutral liposomes (C). In the presence of BMP (D), it is enhanced strongly by DAG and slightly by MAG and stearic acid, but strongly inhibited by sphingosine and sphinganine. The effects of lyso-PC depend on the liposome types. The experiments were carried out using 10 mol% each of the stimulating or inhibiting lipids and 400 or 60 ng GBA1 per assay with 1 or 0.5 μg Sap C for neutral and BMP-containing liposomes, respectively, under 30 min incubation and at 37°C. SEM was less than 10% (n = 4–6).
Various lysosomal lipid degradation products influence Sap activity differently
As observed with the GBA1 activity in the absence of Sap C (earlier shown in Fig. 6A–C), various lysosomal lipid degradation products play different roles on the degradation of GlcCer with DAG, MAG, stearic acid, lyso-PA, and lyso-PS playing stimulatory roles, while sphingosine and sphinganine play inhibitory roles. Similar trends were observed in Sap C-induced GlcCer degradation experiments (Fig. 8C), with DAG and stearic acid playing the strongest stimulatory roles (up to 2-fold) and sphingosine and sphinganine exhibiting inhibitory tendencies (up to 50% inhibition) on the Sap C activity in the absence of BMP. Lyso-PC, which plays an insignificant role on GBA1 activity in the absence of Sap C, strongly attenuates the activity in the presence of Sap C. In the presence of BMP, only DAG enhances Sap C activity up to 3-fold, while the roles played by MAG and stearic acid are relatively insignificant (Fig. 8D). Lyso-PC weakly inhibits Sap C activity, whereas a usually strong inhibition (up to 60%) was exhibited by the positively charged sphingosine and sphinganine in the presence of BMP.
DISCUSSION
Organelles of eukaryotic cells maintain an organelle-specific lipid pattern of their surrounding and limiting membranes, which seems to be in a steady state situation between lipid biosynthesis and degradation, as well as the lipid trafficking into and out of the organellar membranes. For instance, sphingolipids (glycosphingolipids and SM) and cholesterol are enriched in plasma membranes where they confer high stability on the membranes with high cholesterol content functioning to regulate the functional properties of Na+-K+ ATPase and to lower the permeability of the sodium ions, thus allowing for successful electrochemical gradients (49). But their contents are low in the lysosomal compartments of healthy cells. Imbalances of membrane lipid profiles may well affect organellar functions, as observed in Niemann-Pick disease types A, B, and C (25, 41). High SM storage in endolysosomes of Niemann-Pick disease types A and B patients inhibit cholesterol egress from the late endosomes by NPC2 (18), thereby leading to the secondary accumulation of cholesterol in the lysosomes of the patients (25). Similarly, cholesterol accumulation in Niemann-Pick disease type C patients inhibits the catabolism of GM2 and the activities of several sphingolipid activator proteins (22, 38, 39), thus triggering the secondary storage of glycolipids in Niemann-Pick disease type C patients (24).
GBA1 activity is essential for GlcCer degradation, with its functional defects leading to Gaucher disease, of which three clinical variants (types I, II, and III) are known (2). However, there are poor correlations between the genotypes and phenotypes of patients affected by this disease. Strong modifiers of GlcCer catabolism (membrane lipids and Sap C) have not been properly addressed in previous reports, which employed only the use of synthetic soluble substrates to assay the activity of GBA1. To address this, a detergent-free liposomal assay, which employed the natural lipid substrate, radiolabeled GlcCer, and mimics the lysosomal lipid composition and vesicle sizes as closely as possible, was developed in our laboratory (11).
Previous work in our laboratory showed how GlcCer hydrolysis was stimulated by various membrane lipids using a liposomal assay (11) based on the knowledge of lipid compositions of cellular compartments that were published at the time. However, the liposomal cholesterol content employed was much higher than what was recently reported for lysosomes. For example, recent works in other laboratories indicate that purified lysosomes contain only traces of cholesterol, as low as 5 mol% (13, 14), being relatively low compared with the liposomal content (23 mol%) used in the previously reported work (11). Hence, a need arises to reevaluate how GlcCer is degraded under a condition mimicking the topology of the intralysosomal vesicles as closely as possible, using a modified detergent-free liposomal assay at lower cholesterol contents.
GlcCer hydrolysis is modulated by the microenvironment of the enzymatic reaction
In vitro experiments demonstrate that GlcCer, which is membrane bound, is presumably degraded as a membrane component of endolysosomal vesicles by the soluble and membrane surface-associated GBA1. As shown in this work, components of the organellar environment (pH, ionic strength, incubation time, and presence of anionic lipids, among others) modify the enzymatic reaction rates. Low pH and low ionic strength favor the degradation, thus corroborating the fact that GlcCer and other glycosphingolipids are degraded by GBA1 within the lumen of the acidic compartments of the cell as components of intraendosomal and intralysosomal vesicles (44, 50). The ionic strength of a solution is an important parameter affecting enzyme activity. The activities of a number of lysosomal enzymes, such as lysosomal phospholipase A2 (36) or hexosaminidase A, in the presence of GM2 activator protein (22) are strongly inhibited at high salt concentrations. Similarly, high ionic strength could impair the ability of GBA1 to degrade GlcCer (Fig. 1E, F), which could lead to the accumulation of the latter in the lysosomes. Ionic strength, together with other factors, such as the net charge of the enzyme and the membranes at a defined pH, is a great factor that affects electrostatic interactions, a great necessity for the lipid-protein optimum association.
The extent of GlcCer hydrolysis is dramatic, as PA and other ionic lipids strongly stimulate the hydrolysis, with PA having a stimulatory power up to three orders of magnitude over neutral liposomes, even in the absence of Sap C, in agreement with previous observations (11). Anionic lipids create negative surface charges on the liposomes, which attract the cationic protein (GBA1) at low pH, thus making the enzyme electrostatically bind to the anionic surfaces of the luminal vesicles, but these electrostatic interactions are disturbed by the addition of cationic lipids and also by increasing ionic strength, as observed in this work. This strong role of electrostatic interactions has been confirmed previously in our laboratory, as with the case of the association between acid sphingomyelinase and different anionic and cationic membranes at pH 5 through zeta potential measurements (21). It appears to be a general phenomenon that anionic lipids, like BMP and others, create a negative surface potential on the surfaces of LVs, which helps to attract and concentrate protonated and cationic lysosomal hydrolases and SAPs on vesicle surfaces to facilitate sphingolipid and membrane digestion. The observation that the anionic lipids (PA, PG, sulfatide, BMP, PI, PS, GM1, GM2, and GM3) found in different cellular compartments stimulate the enzymatic rate to physiologically significant levels, even in the absence of Sap C, supports the previous report on GlcCer hydrolysis stimulation by anionic lipids in our laboratory (11). Also of great importance is the ability of PA to stimulate GlcCer degradation at higher pH (even at pH >6). This could facilitate the degradation of GlcCer derived from endocytic and autophagic processes, even before reaching the acidic lysosomal compartments. However, the presence of the common lysosomal enzyme activity inhibitor lipid (SM), which is degraded at a slightly lower pH of 5 to Cer by acid sphingomyelinase, could inhibit the PA-stimulated GlcCer degradation at the higher pH. This proves further that the lipid compositions of various cellular microenvironments play major regulatory roles.
Although PA and some of these anionic lipids are subjected to degradation by their respective lysosomal enzymes, BMP, which is a unique anionic lipid derived within the luminal membranes of intraendolysosomal vesicles as an intermediate of PG catabolism during the endocytic process, is quite stable against degradation at this low pH. The resistance of BMP to the action of endolysosomal phospholipases is associated to its unusual sn1, sn1′ configuration. Negatively charged BMP, which stimulates most of the catabolic processes in the lysosome, is exclusively formed in the intravesicular membranes (6, 13, 51), but not in the lysosomal perimeter membrane, which seems to be quite resistant to lysosomal digestion. This is so because the perimeter membrane is protected by a thick glycocalix on its luminal leaflet from the attack by membrane-degrading enzymes present in the lumen of the lysosome (52–56). This lysosomal perimeter membrane has a lateral pressure of at least 31 mN/m, too high to be attacked by GM2 activator protein (57) or any other sphingolipid activator protein, as GM2 activator protein can only insert into the monolayer and reaches the air-water interface below a monolayer pressure of 25 mN/m, depending on the lipid compositions (57).
The presence of BMP above a threshold concentration around 20 mol% is needed to stimulate a substantial amount of GlcCer degradation (Fig. 3). This is easily achieved in the lysosomes, as BMP could reach up to 30–60 mol% of the total phospholipids in the lysosomal internal membranes (13, 20). With this, no Sap C may be required for the GlcCer hydrolysis, although Sap C could compensate for the case in which no or low BMP content is present. The almost sigmoidal curve obtained with BMP-stimulated GlcCer hydrolysis (Fig. 3) is similar to observations obtained on the regulation of the mitochondrial cardiolipin synthase by phosphatidylethanolamine (58) and the regulation of the mitochondrial 3-hydroxybutyrate dehydrogenase by phosphatidylcholine (59). This depicts that cooperativity exists between BMP-containing liposomes and GBA1, and these observations and others showed the roles played by the organellar microenvironment on the enzymatic reactions taking place in various cellular organelles.
Cer, SM, cholesterol, and lysosomal lipid degradation products are strong modifiers of GlcCer hydrolysis
In the secondary accumulation of lipids like cholesterol, SM, phospholipids, and glycosphingolipids in various lysosomal disorders, different patterns of lipid accumulation in brain and in visceral organs often occur. For example, the deficiency in or inactivity of acid sphingomyelinase, as observed in Niemann-Pick disease types A and B incidences, leads to a primary accumulation of SM that acts as an inhibitory lipid in various endolysosomal processes. It inhibits cholesterol transport by NPC2 (18) as well as GM2 degradation (22). These observations could therefore explain the mechanism by which a substantial secondary accumulation of cholesterol and GM2 in the endolysosomal compartments of Niemann-Pick disease types A and B patients takes place (25). Although cholesterol is the primary lipid storage in Niemann-Pick disease type C, SM also accumulates secondarily and this explains the complex nature of secondary accumulation of other lipids in Niemann-Pick disease type C too. The similar inhibitory effect of SM on GlcCer degradation observed in this work could elucidate why the GlcCer also accumulates secondarily in Niemann-Pick disease types A, B, and C incidences (60, 61). Therefore, as a precondition for the clearance of GlcCer accumulation in Niemann-Pick disease types A and B incidences, hydrolysis of vesicular SM to Cer by active acid sphingomyelinase at a pH of about 5 (21) has to take place because the degradation product, Cer, also acts as a strong stimulator of GlcCer hydrolysis at low pH, especially in the presence of BMP. The degradation of SM into Cer by acid sphingomyelinase is very important in enhancing the degradation of GlcCer. To mimic the action of acid sphingomyelinase, we replaced SM with Cer in our in vitro assay, which strongly stimulated GlcCer hydrolysis by up to 500-fold (Fig. 4D). The ability of Cer to strongly stimulate GlcCer hydrolysis could be due to its ability to induce transbilayer motion of other lipids, and bilayer scrambling (62, 63). This flip-flop movement could easily make the GlcCer more accessible for degradation by GBA1. However, Cer may behave differently in an environment with different lipid compositions. For example, a previous work (12) that employed the use of PA instead of BMP as the anionic lipid reported Cer as an inhibitor at high cholesterol content at a pH of 5. Our work also affirms that Cer and cholesterol may not play any significant role if BMP is replaced with PA in the liposomal assay at low cholesterol concentration (Fig. 5), although the strong inhibitory role of SM is sustained. This further confirms that cellular processes are complex and highly regulated by the nature of the membrane lipids found in different cellular organelles.
Neuronal plasma membranes are enriched with cholesterol, as high as 40 mol%, as well as SM and gangliosides, thus conferring high stability on the membranes. The high cholesterol of plasma membrane functions to regulate the functional properties of many membrane bound proteins, such as Na+-K+ ATPase, in which high cholesterol content helps to lower the permeability of the sodium ions, thus allowing for successful electrochemical gradients (49), and might be important for several membrane interaction processes. Cholesterol has also been reported to be an activator of the membrane protein, smoothened, in hedgehog signaling, a pathway necessary and critical in embryogenesis and cancer (64). However, cholesterol concentration is drastically reduced before reaching the lysosomes, as it is transported out of the intraluminal vesicles of the late endosomes by NPC1 and NPC2 proteins (13). Deficiency in either of these two proteins leads to the high accumulation of unesterified cholesterol that is observed in Niemann-Pick disease type C, which is characterized by the secondary accumulation of SM, GM2, and GM3, as well as less complex glycolipids, most importantly lactosylceramide and GlcCer (37, 65). The secondary accumulation of GM2 in Niemann-Pick disease type C patients was attributed to the inhibitory roles played by cholesterol on GM2AP-induced GM2 hydrolysis (22) and this could similarly account for GM3 secondary accumulation. Surprisingly, cholesterol plays a strong stimulatory role (rather than the much more common inhibitory roles it is known for) on GlcCer hydrolysis, as observed in this work (Fig. 4A), most especially in the presence of BMP. The stimulatory effect of cholesterol is of special interest because GBA1 has been reported to generate glucosylated cholesterol by transglucosylation of cholesterol with GlcCer as glucose donor, a reaction strongly promoted by increasing intralysosomal cholesterol levels, as observed in Niemann-Pick disease type C and pharmacologically induced by U186333A in cultured cells (66). Nevertheless, once cholesterol accumulates in Niemann-Pick disease type C, the activities of several sphingolipid activator proteins are inhibited (22, 38, 39) and acid sphingomyelinase activity is reduced (60, 65, 67) leading to a secondary accumulation of SM. This secondary SM accumulation effected by loss of ASM activity at high cholesterol may, in turn, inhibit GBA1 activity strongly, thus triggering the GlcCer secondary accumulation, as observed in the lysosomes of Niemann-Pick disease type C patients (37, 65).
Sphinganine and sphingosine are two free sphingoid bases with large increasing amounts reported in the livers of Niemann-Pick disease type C patients (68–70); their pathomechanisms in lysosomal storage disorders have been reviewed previously (24). Sphinganine and sphingosine are also strong inhibitors of GlcCer hydrolysis, as observed in this work as well in a previous work (12) that showed that their inhibitory roles could be associated with the positive charges on sphingoid bases. They can reduce the strong electrostatic interactions between the cationic enzyme and the negative surface charges on the membrane effected by the anionic lipids like BMP at low pH values. The mechanism involved may be similar to that in drug-induced lipidosis, as reported earlier for acid sphingomyelinase (67, 71). These highly cytotoxic sphingoid bases have been reported to be closely associated to the primary defect in several lysosomal lipid storage diseases, such as Krabbe disease (72, 73) and Fabry disease (74), as well as Niemann-Pick disease type A (75).
The degradation of most phospholipids occurs in the endolysosomal compartments by the action of endolysosomal phospholipases, thus leading to the production of the intermediates, such as MAG, DAG, fatty acids, and lysolipids, carrying different charges corresponding to those on the head groups of their main degrading lipids. An increasing DAG content has been previously reported to trigger SM cleavage by acid sphingomyelinase, both in the presence and absence of anionic lipids in the liposomal assay (21), as well in the micellar assay in the presence or absence of detergents (76, 77). Similar stimulation of GlcCer hydrolysis by DAG, mostly in the presence of BMP, observed in this work may be attributed to its ability to modulate membrane structure. However, SM hydrolysis stimulation was enhanced strongly by DAG, even at low contents and in the absence of anionic lipids (21), contrary to that observed for GlcCer hydrolysis in the present work. GlcCer hydrolysis stimulation by DAG could be a result of its ability to effect a change from lamellar to hexagonal structure in the bilayer, as explained in previous biophysical studies in model membranes (78, 79), depending on its contents in the membrane. It is also involved in membrane fusion and fission processes (80, 81). The presence of MAG and fatty acids in the lysosomes as lipid degradation products could also aid the hydrolysis of GlcCer by GBA1, especially in the presence of BMP, as observed in this work. The stimulation of GlcCer hydrolysis by fatty acids in the neutral liposomes may be attributable to their negative charges at low physiological pH. However, the strong stimulation of GlcCer hydrolysis by lysolipids with negatively charged head groups, lyso-PA and lyso-PS, compared with the zwitterionic lyso-PC further confirms the strong effects that electrostatic interactions between GBA1 and negative charges on the membranes may have on GlcCer hydrolysis.
Sap C is essential for enzymatic GlcCer hydrolysis embedded in neutral membranes
Sap C, one of the four homologous proteins (others being Saps A, B, and D) derived from the sequential cleavage of prosaposin (Sap precursor protein), is an essential cofactor that enhances the activity of GBA1 in GlcCer hydrolysis. Its deficiency causes an abnormal juvenile type of Gaucher disease characterized by GlcCer accumulation in the lysosomes (7, 8). Our observations agreed with the previous in vitro studies that have shown that anionic lipids could stimulate GlcCer hydrolysis, to some extent, depending on the nature of the head groups, even in the absence of Sap C (11, 12, 47, 82, 83).
As shown in this work, high GlcCer degradation could have taken place already in the absence of Sap C once adequate lysosomal BMP concentration (probably ≥20 mol%) was reached, which is physiologically possible (20) keeping other inhibiting factors constant. However, in cases where BMP is completely absent (neutral membranes) or its negative charges and those of other anionic membrane lipids have been countered by the positive charges of sphingoid bases, Sap C could still significantly stimulate GlcCer hydrolysis. The further enhancement in GlcCer hydrolysis by Sap C in the absence or presence of BMP by the presence of Cer, cholesterol, and other lysosomal degradation products as well its inhibition by SM, sphingosine, sphinganine, and lyso-PC could explain further the extent to which membrane lipids modulate the functional properties of protein cofactors in the lysosome. More importantly, Sap C does not require BMP to stimulate a substantial amount of GlcCer hydrolysis at a moderately higher GBA1 concentration. However, only a little, but not substantial, further stimulation of GlcCer hydrolysis could be displayed by Sap C (except at high concentrations) in the presence of PA, as reported previously (11).
The exact mode of action of Sap C in enhancing the hydrolysis of GlcCer by GBA1 is yet to be fully unraveled. While some findings implicated Saps (like Sap C) in glycolipid antigen presentation by CD1 (84–86), others suggested that the target lipid molecules are extracted from the bilayers by Saps (such as Saps A and B), become solubilized, and are then presented to the respective enzymes as soluble protein-lipid complexes (38, 39, 87). But in all, Sap C has been reported to interact with GBA1 (87) and bind the enzyme to the bilayer surface (11, 33), thereby facilitating the access to the GlcCer substrate. The reported binding of Sap C to GBA1 (88) may well change the properties of GBA1 and thus also increase its activity toward the water soluble artificial substrate, MUG, which is also increased by anionic lipids (11, 12). Does Sap C act as a lipid transfer protein or a lipid solubilizer or does it merely participate in the vesicle fusion? These questions necessitate further research to elucidate the clear roles played by Sap C in GlcCer hydrolysis by GBA1.
CONCLUSIONS
A modified detergent-free liposomal assay to measure the regulatory roles of some lysosomal lipids on membrane bound GlcCer degradation was optimized. GlcCer hydrolysis is modulated by the microenvironment of the enzymatic reaction with the hydrolysis favored at low pH and low ionic strength. BMP, which is generated in the intraluminal vesicle of endolysosomes, as well as other anionic lipids, strongly stimulates GlcCer hydrolysis. Cer, DAG, MAG, fatty acids, and, surprisingly, cholesterol all exhibit stimulatory effects on GlcCer hydrolysis, while SM, sphingosine, and sphinganine play inhibitory roles. Concentrations of BMP and other lipids in the lysosomal compartment may vary tremendously between different cell types, and also between lysosomes of one and the same cell due to local fluctuations between influx rates into individual lysosomes and catabolic rates of lipids within individual lysosomes. Local lysosomal conditions may be even more critical for some mutant lysosomal hydrolases, e.g., for mutant GBA1 (89). In Niemann-Pick type C disease, characterized by the cholesterol primary storage, GlcCer secondary accumulation could be triggered by SM secondary accumulation. Sap C stimulates GlcCer hydrolysis even in the absence of BMP, although an adequate BMP concentration may not necessitate the requirement of Sap C. All these strong modifiers of GlcCer hydrolysis may well affect the genotype-phenotype correlation in several cases for Gaucher patients independent of the types, whether in neuropathic infantile and juvenile patients or in nonneuropathic adult patients.
Acknowledgments
The authors thank Dr. Ellen Sidransky (National Institutes of Health, Molecular Neurogenetics Section) for her gift of a sample of Gaucher spleen. We acknowledge the technical assistance of Jennifer Mainzer for Sap C purification.
Footnotes
Abbreviations:
- BMP
- bis(monoacylglycero)phosphate
- Cer
- ceramide
- DAG
- diacylglycerol
- DHP
- dihexadecyl phosphate
- DOPC
- dioleoyl-l-α-phosphatidylcholine
- DOTMA
- 1,2-di-O-octadecenyl-3-trimethylammonium propane
- EPC
- 1,2-dioleoyl-sn-glycero-3-ethylphosphocholine
- GBA1
- lysosomal β-glucocerebrosidase
- GBA2
- non-lysosomal β-glucocerebrosidase
- GlcCer
- glucosylceramide
- lyso-PC
- lysophosphatidylcholine
- MAG
- monoacylglycerol
- MUG
- 4-methylumbelliferyl-β-D-glucopyranoside
- MVL5
- multivalent cationic lipid
- NPC1
- Niemann-Pick disease type C1 protein
- NPC2
- Niemann-Pick disease type C2 protein
- PA
- phosphatidic acid
- PG
- phosphatidylglycerol
- PI
- phosphatidylinositol
- PS
- phosphatidylserine
- Sap
- saposin
This work was supported by German Research Foundation Grants SFB 645 and TRR 83, and Fonds der Chemischen Industrie.
REFERENCES
- 1.Schulze H., Kolter T., and Sandhoff K.. 2009. Principles of lysosomal membrane degradation: Cellular topology and biochemistry of lysosomal lipid degradation. Biochim. Biophys. Acta. 1793: 674–683. [DOI] [PubMed] [Google Scholar]
- 2.Brady R. O., Kanfer J. N., and Shapiro D.. 1965. Metabolism of glucocerebrosides. II. Evidence of an enzymatic deficiency in Gaucher’s disease. Biochem. Biophys. Res. Commun. 18: 221–225. [DOI] [PubMed] [Google Scholar]
- 3.Eckford P. D., and Sharom F. J.. 2005. The reconstituted P-glycoprotein multidrug transporter is a flippase for glucosylceramide and other simple glycosphingolipids. Biochem. J. 389: 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasaki T. 1990. Glycolipid transfer protein and intracellular traffic of glucosylceramide. Experientia. 46: 611–616. [DOI] [PubMed] [Google Scholar]
- 5.Möbius W., Herzog V., Sandhoff K., and Schwarzmann G.. 1999. Intracellular distribution of a biotin-labeled ganglioside, GM1, by immunoelectron microscopy after endocytosis in fibroblasts. J. Histochem. Cytochem. 47: 1005–1014. [DOI] [PubMed] [Google Scholar]
- 6.Kolter T., and Sandhoff K.. 2005. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu. Rev. Cell Dev. Biol. 21: 81–103. [DOI] [PubMed] [Google Scholar]
- 7.Qi X., and Grabowski G. A.. 2001. Molecular and cell biology of acid beta-glucosidase and prosaposin. Prog. Nucleic Acid Res. Mol. Biol. 66: 203–239. [DOI] [PubMed] [Google Scholar]
- 8.Tylki-Szymańska A., Czartoryska B., Vanier M. T., Poorthuis B., Groener J. A. E., Ługowska A., Millat G., Vaccaro A. M., and Jurkiewicz E.. 2007. Non-neuronopathic Gaucher disease due to saposin C deficiency. Clin. Genet. 72: 538–542. [DOI] [PubMed] [Google Scholar]
- 9.van Weely S., Brandsma M., Strijland A., Tager J. M., and Aerts J. M. F. G.. 1993. Demonstration of the existence of a second, non-lysosomal glucocerebrosidase that is not deficient in Gaucher disease. Biochim. Biophys. Acta. 1181: 55–62. [DOI] [PubMed] [Google Scholar]
- 10.Yildiz Y., Hoffmann P., vom Dahl S., Breiden B., Sandhoff R., Niederau C., Horwitz M., Karlsson S., Filacamo M., Elstein D., et al. . 2013. Functional and genetic characterization of the non-lysosomal glucosylceramidase 2 as a modifier for Gaucher disease. Orphanet J. Rare Dis. 8: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkening G., Linke T., and Sandhoff K.. 1998. Lysosomal degradation on vesicular membrane surfaces. Enhanced glucosylceramide degradation by lysosomal anionic lipids and activators. J. Biol. Chem. 273: 30271–30278. [DOI] [PubMed] [Google Scholar]
- 12.Sarmientos F., Schwarzmann G., and Sandhoff K.. 1986. Specificity of human glucosylceramide beta-glucosidase towards synthetic glucosylsphingolipids inserted into liposomes. Kinetic studies in a detergent-free assay system. Eur. J. Biochem. 160: 527–535. [DOI] [PubMed] [Google Scholar]
- 13.Möbius W., van Donselaar E., Ohno-Iwashita Y., Shimada Y., Heijnen H. F., Slot J. W., and Geuze H. J.. 2003. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 4: 222–231. [DOI] [PubMed] [Google Scholar]
- 14.Schoer J. K., Gallegos A. M., McIntosh A. L., Starodub O., Kier A. B., Billheimer J. T., and Schroeder F.. 2000. Lysosomal membrane cholesterol dynamics. Biochemistry. 39: 7662–7677. [DOI] [PubMed] [Google Scholar]
- 15.Karten B., Peake K. B., and Vance J. E.. 2009. Mechanisms and consequences of impaired lipid trafficking in Niemann-Pick type C1-deficient mammalian cells. Biochim. Biophys. Acta. 1791: 659–670. [DOI] [PubMed] [Google Scholar]
- 16.Storch J., and Xu Z.. 2009. Niemann-Pick C2 (NPC2) and intracellular cholesterol trafficking. Biochim. Biophys. Acta. 1791: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vance J. E. 2010. Transfer of cholesterol by the NPC team. Cell Metab. 12: 105–106. [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Hammed M., Breiden B., Adebayo M. A., Babalola J. O., Schwarzmann G., and Sandhoff K.. 2010. Role of endosomal membrane lipids and NPC2 in cholesterol transfer and membrane fusion. J. Lipid Res. 51: 1747–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Z., Farver W., Kodukula S., and Storch J.. 2008. Regulation of sterol transport between membranes and NPC2. Biochemistry. 47: 11134–11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi T., Beuchat M. H., Chevallier J., Makino A., Mayran N., Escola J. M., Lebrand C., Cosson P., Kobayashi T., and Gruenberg J.. 2002. Separation and characterization of late endosomal membrane domains. J. Biol. Chem. 277: 32157–32164. [DOI] [PubMed] [Google Scholar]
- 21.Oninla V. O., Breiden B., Babalola J. O., and Sandhoff K.. 2014. Acid sphingomyelinase activity is regulated by membrane lipids and facilitates cholesterol transfer by NPC2. J. Lipid Res. 55: 2606–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anheuser S., Breiden B., Schwarzmann G., and Sandhoff K.. 2015. Membrane lipids regulate ganglioside GM2 catabolism and GM2 activator protein activity. J. Lipid Res. 56: 1747–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walkley S. U., and Vanier M. T.. 2009. Secondary lipid accumulation in lysosomal disease. Biochim. Biophys. Acta. 1793: 726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanier M. T. 2015. Complex lipid trafficking in Niemann-Pick disease type C. J. Inherit. Metab. Dis. 38: 187–199. [DOI] [PubMed] [Google Scholar]
- 25.Vanier M. T. 1983. Biochemical studies in Niemann-Pick disease I. Major sphingolipids of liver and spleen. Biochim. Biophys. Acta. 750: 178–184. [DOI] [PubMed] [Google Scholar]
- 26.Schwarzmann G., and Sandhoff K.. 1987. Lysogangliosides: synthesis and use in preparing labeled gangliosides. Methods Enzymol. 138: 319–341. [DOI] [PubMed] [Google Scholar]
- 27.Sarmientos F., Schwarzmann G., and Sandhoff K.. 1985. Direct evidence by carbon-13 NMR spectroscopy for the erythro configuration of the sphingoid moiety in Gaucher cerebroside and other natural sphingolipids. Eur. J. Biochem. 146: 59–64. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro D., Rachaman E. S., and Sheradsky T.. 1964. Synthetic studies on sphingolipids. X. Synthesis of psychosine. J. Am. Chem. Soc. 86: 4472–4476. [Google Scholar]
- 29.Schwarzmann G., Breiden B., and Sandhoff K.. 2015. Membrane-spanning lipids for an uncompromised monitoring of membrane fusion and intermembrane lipid transfer. J. Lipid Res. 56: 1861–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Körschen H. G., Yildiz Y., Raju D. N., Schonauer S., Bönigk W., Jansen V., Kremmer E., Kaupp U. B., and Wachten D.. 2013. The non-lysosomal β-glucosidase GBA2 is a non-integral membrane-associated protein at the endoplasmic reticulum (ER) and Golgi. J. Biol. Chem. 288: 3381–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto S., Martin B. M., Yamamoto Y., Kretz K. A., O’Brien J. S., and Kishimoto Y.. 1989. Saposin A: second cerebrosidase activator protein. Proc. Natl. Acad. Sci. USA. 86: 3389–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald R. C., MacDonald R. I., Menco B. P. M., Takeshita K., Subbarao N. K., and Hu L-r.. 1991. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta. 1061: 297–303. [DOI] [PubMed] [Google Scholar]
- 33.Kolter T., and Sandhoff K.. 2010. Lysosomal degradation of membrane lipids. FEBS Lett. 584: 1700–1712. [DOI] [PubMed] [Google Scholar]
- 34.Hinton A., Bond S., and Forgac M.. 2009. V-ATPase functions in normal and disease processes. Pflugers Arch. 457: 589–598. [DOI] [PubMed] [Google Scholar]
- 35.Rome L. H., and Crain L. R.. 1981. Degradation of mucopolysaccharide in intact isolated lysosomes. J. Biol. Chem. 256: 10763–10768. [PubMed] [Google Scholar]
- 36.Abe A., and Shayman J. A.. 2009. The role of negatively charged lipids in lysosomal phospholipase A2 function. J. Lipid Res. 50: 2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zervas M., Dobrenis K., and Walkley S. U.. 2001. Neurons in Niemann-Pick disease type C accumulate gangliosides as well as unesterified cholesterol and undergo dendritic and axonal alterations. J. Neuropathol. Exp. Neurol. 60: 49–64. [DOI] [PubMed] [Google Scholar]
- 38.Locatelli-Hoops S., Remmel N., Klingenstein R., Breiden B., Rossocha M., Schoeniger M., Koenigs C., Saenger W., and Sandhoff K.. 2006. Saposin A mobilizes lipids from low cholesterol and high bis(monoacylglycerol)phosphate-containing membranes: patient variant Saposin A lacks lipid extraction capacity. J. Biol. Chem. 281: 32451–32460. [DOI] [PubMed] [Google Scholar]
- 39.Remmel N., Locatelli-Hoops S., Breiden B., Schwarzmann G., and Sandhoff K.. 2007. Saposin B mobilizes lipids from cholesterol-poor and bis(monoacylglycero)phosphate-rich membranes at acidic pH. Unglycosylated patient variant saposin B lacks lipid-extraction capacity. FEBS J. 274: 3405–3420. [DOI] [PubMed] [Google Scholar]
- 40.Linke T., Wilkening G., Lansmann S., Moczall H., Bartelsen O., Weisgerber J., and Sandhoff K.. 2001. Stimulation of acid sphingomyelinase activity by lysosomal lipids and sphingolipid activator proteins. Biol. Chem. 382: 283–290. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert E. F., Callahan J., Viseskul C., and Opitz J. M.. 1981. Niemann-Pick disease type C. Pathological, histochemical, ultrastructural and biochemical studies. Eur. J. Pediatr. 136: 263–274. [DOI] [PubMed] [Google Scholar]
- 42.Slotte J. P., Hedstrom G., Rannstrom S., and Ekman S.. 1989. Effects of sphingomyelin degradation on cell cholesterol oxidizability and steady-state distribution between the cell surface and the cell interior. Biochim. Biophys. Acta. 985: 90–96. [DOI] [PubMed] [Google Scholar]
- 43.Klein A., Henseler M., Klein C., Suzuki K., Harzer K., and Sandhoff K.. 1994. Sphingolipid activator protein D (sap-D) stimulates the lysosomal degradation of ceramide in vivo. Biochem. Biophys. Res. Commun. 200: 1440–1448. [DOI] [PubMed] [Google Scholar]
- 44.Fürst W., and Sandhoff K.. 1992. Activator proteins and topology of lysosomal sphingolipid catabolism. Biochim. Biophys. Acta. 1126: 1–16. [DOI] [PubMed] [Google Scholar]
- 45.Vaccaro A. M., Salvioli R., Barca A., Tatti M., Ciaffoni F., Maras B., Siciliano R., Zappacosta F., Amoresano A., and Pucci P.. 1995. Structural analysis of saposin C and B. J. Biol. Chem. 270: 9953–9960. [DOI] [PubMed] [Google Scholar]
- 46.Vaccaro A. M., Tatti M., Ciaffoni F., Salvioli R., Barca A., and Scerch C.. 1997. Effect of saposins A and C on the enzymatic hydrolysis of liposomal glucosylceramide. J. Biol. Chem. 272: 16862–16867. [DOI] [PubMed] [Google Scholar]
- 47.Morimoto S., Kishimoto Y., Tomich J., Weiler S., Ohashi T., Barranger J. A., Kretz K. A., and O’Brien J. S.. 1990. Interaction of saposins, acidic lipids, and glucosylceramidase. J. Biol. Chem. 265: 1933–1937. [PubMed] [Google Scholar]
- 48.Wilkening G., Linke T., Uhlhorn-Dierks G., and Sandhoff K.. 2000. Degradation of membrane-bound ganglioside GM1. Stimulation by bis(monoacylglycero)phosphate and the activator proteins SAP-B and GM2-AP. J. Biol. Chem. 275: 35814–35819. [DOI] [PubMed] [Google Scholar]
- 49.Mouritsen O. G., and Zuckermann M. J.. 2004. What’s so special about cholesterol? Lipids. 39: 1101–1113. [DOI] [PubMed] [Google Scholar]
- 50.Sandhoff K., and Kolter T.. 2003. Biosynthesis and degradation of mammalian glycosphingolipids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358: 847–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallala H. D., Breiden B., and Sandhoff K.. 2011. Regulation of the NPC2 protein-mediated cholesterol trafficking by membrane lipids. J. Neurochem. 116: 702–707. [DOI] [PubMed] [Google Scholar]
- 52.Sandhoff K. 2012. My journey into the world of sphingolipids and sphingolipidoses. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 88: 554–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eskelinen E-L., Tanaka Y., and Saftig P.. 2003. At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13: 137–145. [DOI] [PubMed] [Google Scholar]
- 54.Carlsson S. R., and Fukuda M.. 1990. The polylactosaminoglycans of human lysosomal membrane glycoproteins lamp-1 and lamp-2. Localization on the peptide backbones. J. Biol. Chem. 265: 20488–20495. [PubMed] [Google Scholar]
- 55.Kornfeld S., and Mellman I.. 1989. The biogenesis of lysosomes. Annu. Rev. Cell Biol. 5: 483–525. [DOI] [PubMed] [Google Scholar]
- 56.Henning R., and Stoffel W.. 1973. Glycosphingolipids in lysosomal membranes. Hoppe Seylers Z. Physiol. Chem. 354: 760–770. [DOI] [PubMed] [Google Scholar]
- 57.Giehl A., Lemm T., Bartelsen O., Sandhoff K., and Blume A.. 1999. Interaction of the GM2-activator protein with phospholipid-ganglioside bilayer membranes and with monolayers at the air-water interface. Eur. J. Biochem. 261: 650–658. [DOI] [PubMed] [Google Scholar]
- 58.Schlame M., and Hostetler K. Y.. 1991. Solubilization, purification, and characterization of cardiolipin synthase from rat liver mitochondria. Demonstration of its phospholipid requirement. J. Biol. Chem. 266: 22398–22403. [PubMed] [Google Scholar]
- 59.Sandermann H. Jr., McIntyre J. O., and Fleischer S.. 1986. Site-site interaction in the phospholipid activation of D-beta-hydroxybutyrate dehydrogenase. J. Biol. Chem. 261: 6201–6208. [PubMed] [Google Scholar]
- 60.Devlin C., Pipalia N. H., Liao X., Schuchman E. H., Maxfield F. R., and Tabas I.. 2010. Improvement in lipid and protein trafficking in NPC1 cells by correction of a secondary enzyme defect. Traffic. 11: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mukherjee S., and Maxfield F. R.. 2004. Lipid and cholesterol trafficking in NPC. Biochim. Biophys. Acta. 1685: 28–37. [DOI] [PubMed] [Google Scholar]
- 62.Contreras F. X., Sanchez-Magraner L., Alonso A., and Goni F. M.. 2010. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Lett. 584: 1779–1786. [DOI] [PubMed] [Google Scholar]
- 63.Contreras F. X., Villar A. V., Alonso A., and Goni F. M.. 2009. Ceramide-induced transbilayer (flip-flop) lipid movement in membranes. Methods Mol. Biol. 462: 155–165. [DOI] [PubMed] [Google Scholar]
- 64.Huang P., Nedelcu D., Watanabe M., Jao C., Kim Y., Liu J., and Salic A.. 2016. Cellular cholesterol directly activates smoothened in hedgehog signaling. Cell. 166: 1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vanier M. T. 1999. Lipid changes in Niemann-Pick disease type C brain: personal experience and review of the literature. Neurochem. Res. 24: 481–489. [DOI] [PubMed] [Google Scholar]
- 66.Marques A. R., Mirzaian M., Akiyama H., Wisse P., Ferraz M. J., Gaspar P., Ghauharali-van der Vlugt K., Meijer R., Giraldo P., Alfonso P., et al. . 2016. Glucosylated cholesterol in mammalian cells and tissues: formation and degradation by multiple cellular beta-glucosidases. J. Lipid Res. 57: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hurwitz R., Ferlinz K., and Sandhoff K.. 1994. The tricyclic antidepressant desipramine causes proteolytic degradation of lysosomal sphingomyelinase in human fibroblasts. Biol. Chem. Hoppe Seyler. 375: 447–450. [DOI] [PubMed] [Google Scholar]
- 68.te Vruchte D., Lloyd-Evans E., Veldman R. J., Neville D. C., Dwek R. A., Platt F. M., van Blitterswijk W. J., and Sillence D. J.. 2004. Accumulation of glycosphingolipids in Niemann-Pick C disease disrupts endosomal transport. J. Biol. Chem. 279: 26167–26175. [DOI] [PubMed] [Google Scholar]
- 69.Rodriguez-Lafrasse C., Rousson R., Pentchev P. G., Louisot P., and Vanier M. T.. 1994. Free sphingoid bases in tissues from patients with type C Niemann-Pick disease and other lysosomal storage disorders. Biochim. Biophys. Acta. 1226: 138–144. [DOI] [PubMed] [Google Scholar]
- 70.Goldin E., Roff C. F., Miller S. P., Rodriguez-Lafrasse C., Vanier M. T., Brady R. O., and Pentchev P. G.. 1992. Type C Niemann-Pick disease: a murine model of the lysosomal cholesterol lipidosis accumulates sphingosine and sphinganine in liver. Biochim. Biophys. Acta. 1127: 303–311. [DOI] [PubMed] [Google Scholar]
- 71.Kölzer M., Werth N., and Sandhoff K.. 2004. Interactions of acid sphingomyelinase and lipid bilayers in the presence of the tricyclic antidepressant desipramine. FEBS Lett. 559: 96–98. [DOI] [PubMed] [Google Scholar]
- 72.Nilsson O., and Svennerholm L.. 1982. Accumulation of glucosylceramide and glucosylsphingosine (psychosine) in cerebrum and cerebellum in infantile and juvenile Gaucher disease. J. Neurochem. 39: 709–718. [DOI] [PubMed] [Google Scholar]
- 73.Vanier M., and Svennerholm L.. 1976. Chemical pathology of Krabbe disease: the occurrence of psychosine and other neutral sphingoglycolipids. Adv. Exp. Med. Biol. 68: 115–126. [DOI] [PubMed] [Google Scholar]
- 74.Aerts J. M., Groener J. E., Kuiper S., Donker-Koopman W. E., Strijland A., Ottenhoff R., van Roomen C., Mirzaian M., Wijburg F. A., Linthorst G. E., et al. . 2008. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc. Natl. Acad. Sci. USA. 105: 2812–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez-Lafrasse C., and Vanier M. T.. 1999. Sphingosylphosphorylcholine in Niemann-Pick disease brain: accumulation in type A but not in type B. Neurochem. Res. 24: 199–205. [DOI] [PubMed] [Google Scholar]
- 76.Kolesnick R. N., and Clegg S.. 1988. 1,2-Diacylglycerols, but not phorbol esters, activate a potential inhibitory pathway for protein kinase C in GH3 pituitary cells. Evidence for involvement of a sphingomyelinase. J. Biol. Chem. 263: 6534–6537. [PubMed] [Google Scholar]
- 77.Kolesnick R. N. 1987. 1,2-Diacylglycerols but not phorbol esters stimulate sphingomyelin hydrolysis in GH3 pituitary cells. J. Biol. Chem. 262: 16759–16762. [PubMed] [Google Scholar]
- 78.Veiga M. P., Arrondo J. L., Goni F. M., and Alonso A.. 1999. Ceramides in phospholipid membranes: effects on bilayer stability and transition to nonlamellar phases. Biophys. J. 76: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Epand R. M. 1985. Diacylglycerols, lysolecithin, or hydrocarbons markedly alter the bilayer to hexagonal phase transition temperature of phosphatidylethanolamines. Biochemistry. 24: 7092–7095. [DOI] [PubMed] [Google Scholar]
- 80.Basáñez G., Ruiz-Arguello M. B., Alonso A., Goni F. M., Karlsson G., and Edwards K.. 1997. Morphological changes induced by phospholipase C and by sphingomyelinase on large unilamellar vesicles: a cryo-transmission electron microscopy study of liposome fusion. Biophys. J. 72: 2630–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nieva J. L., Alonso A., Basanez G., Goni F. M., Gulik A., Vargas R., and Luzzati V.. 1995. Topological properties of two cubic phases of a phospholipid:cholesterol:diacylglycerol aqueous system and their possible implications in the phospholipase C-induced liposome fusion. FEBS Lett. 368: 143–147. [DOI] [PubMed] [Google Scholar]
- 82.Schnabel D., Schröder M., and Sandhoff K.. 1991. Mutation in the sphingolipid activator protein 2 in a patient with a variant of Gaucher disease. FEBS Lett. 284: 57–59. [DOI] [PubMed] [Google Scholar]
- 83.Vaccaro A. M., Tatti M., Ciaffoni F., Salvioli R., Serafino A., and Barca A.. 1994. Saposin-C induces pH-dependent destabilization and fusion of phosphatidylserine-containing vesicles. FEBS Lett. 349: 181–186. [DOI] [PubMed] [Google Scholar]
- 84.Winau F., Schwierzeck V., Hurwitz R., Remmel N., Sieling P. A., Modlin R. L., Porcelli S. A., Brinkmann V., Sugita M., Sandhoff K., et al. . 2004. Saposin C is required for lipid presentation by human CD1b. Nat. Immunol. 5: 169–174. [DOI] [PubMed] [Google Scholar]
- 85.Zhou D., Cantu C. III, Sagiv Y., Schrantz N., Kulkarni A. B., Qi X., Mahuran D. J., Morales C. R., Grabowski G. A., Benlagha K., et al. . 2004. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. 303: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang S. J., and Cresswell P.. 2004. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat. Immunol. 5: 175–181. [DOI] [PubMed] [Google Scholar]
- 87.Alattia J-R., Shaw J. E., Yip C. M., and Prive G. G.. 2007. Molecular imaging of membrane interfaces reveals mode of beta-glucosidase activation by saposin C. Proc. Natl. Acad. Sci. USA. 104: 17394–17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ho M. W., and O’Brien J. S.. 1971. Gaucher’s disease: deficiency of ‘acid’ -glucosidase and reconstitution of enzyme activity in vitro. Proc. Natl. Acad. Sci. USA. 68: 2810–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Weely S., van den Berg M., Barranger J. A., Sa Miranda M. C., Tager J. M., and Aerts J. M.. 1993. Role of pH in determining the cell-type-specific residual activity of glucocerebrosidase in type 1 Gaucher disease. J. Clin. Invest. 91: 1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]