Figure 4. Protein specificity and secondary structure analysis of HBc particles.
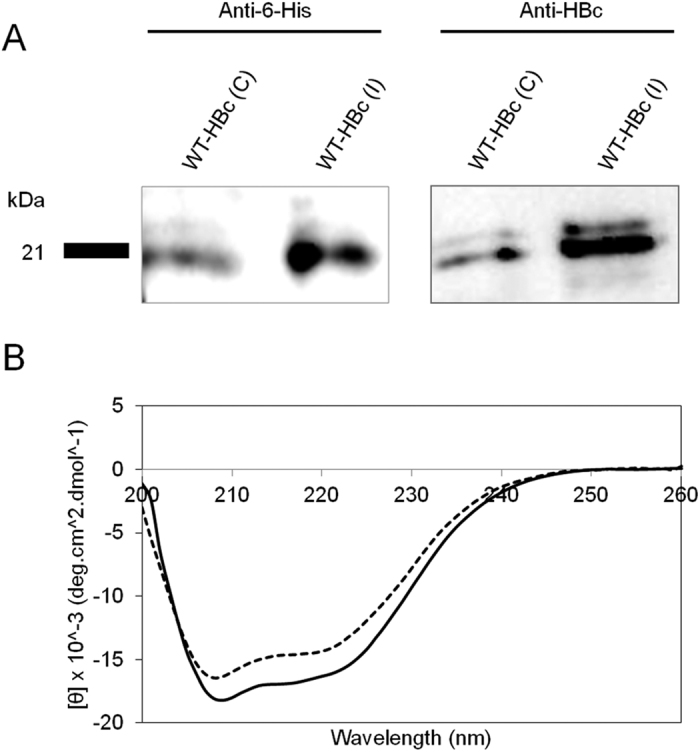
(A) Western blotting (cropped blot) and (B) Circular Dichroism (CD) analysis of WT-HBc particles. Denatured HBc samples were subjected to SDS-PAGE followed by immuno-blotting using anti-6-His and anti-HBc antibodies. Results confirmed the presence of specific protein bands at 21 kDa. CD graph shows the overall conformation of far-UV CD analysis of WT-HBc core particles. WT-HBc (C) (solid line) and WT-HBc (I) (dashed line) exhibited spectra typical of α-helix-containing proteins with minima at 220 and 208 nm. The CD spectra are typical of WT-HBc core monomer secondary structure.
