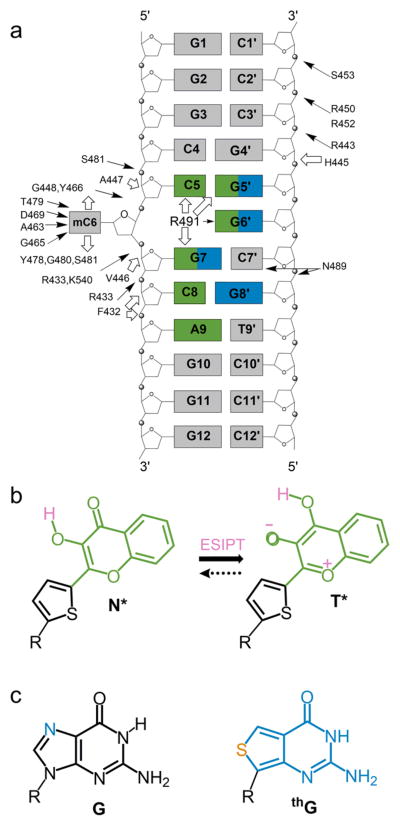
Figure 1.
Structure of the used duplex and fluorescent nucleobase analogues. (a) Structure of the duplex. The interactions of the duplex with SRA, as determined by X-ray crystallography17 are indicated by arrows. Hydrogen bonding and van der Waals interactions are indicated by black and white arrows, respectively. Positions substituted by 3HCnt and thG are highlighted in green and blue, respectively. (b) Structure of the normal (N*) and tautomer (T*) excited-state forms of 3HCnt and ESIPT reaction. (c) Chemical structure of guanosine (G) and its surrogate thienoguanosine (thG).