Abstract
This paper presents a focused review on the nanomaterials and associated transduction schemes that have been developed for the selective detection of hydrogen sulfide. It presents a quite comprehensive overview of the latest developments, briefly discusses the hydrogen sulfide detection mechanisms, identifying the reasons for the selectivity (or lack of) observed experimentally. It critically reviews performance, shortcomings, and identifies missing or overlooked important aspects. It identifies the most mature/promising materials and approaches for achieving inexpensive hydrogen sulfide sensors that could be employed in widespread, miniaturized, and inexpensive detectors and, suggests what research should be undertaken for ensuring that requirements are met.
Keywords: hydrogen sulfide, gas sensor, nanomaterials, air quality monitoring
1. Introduction
Hydrogen sulfide is a gas included in the list of toxic and reactive highly hazardous chemicals from the Occupational Safety and Health Administration (OSHA) [1]. A concentration of 100 ppm of H2S is considered to be immediately dangerous to life and health (IDLH). Since H2S occurs in crude petroleum, natural gas, and hot springs, the main activities in which occupational exposure is likely are petroleum and natural gas drilling, refining, and coke ovens [2,3]. Additionally, since hydrogen sulfide is formed during the decay of organic matter, wastewater treatment plants, landfills, and tanneries are also important emitting sources [4]. Finally, the Kraft process employed in many paper mills, which involves using sodium hydroxide and sodium sulfide also results in the emission of H2S.
Besides the odor nuisance often resulting from hydrogen sulfide emissions, human toxicology data is available, compiled from acute poisoning case reports and occupational exposures. Reported health effects in humans following exposure to hydrogen sulfide include death and respiratory, ocular, neurological, cardiovascular, metabolic, and reproductive effects. The lowest-observed-adverse-effect level (LOAEL) is as low as 2.8 mg/m3 (1.87 ppm) in asthmatic individuals for respiratory and neurological effects [5,6].
The analytical methods most commonly used to measure hydrogen sulfide in air include gas chromatography (GC) or the spectrophotometric method. The latter involves a fixation step in which H2S gas is trapped in a solution that is further made to react with formaldehyde forming a colored product, the absorbance of which is related to hydrogen sulfide concentration [7]. Detection limits reported for the analysis of hydrogen sulfide in air are 10 mg/m3 (6.7 ppm) for GC employing a flame ionization detector, or 0.2 mg/m3 (130 ppb) in spectrophotometry [5]. However, these methods are rather expensive, cumbersome, and not suited for implementing a widespread, continuous monitoring of hydrogen sulfide in ambient conditions. Nowadays, most commercially available hydrogen sulfide detectors employ either electrochemical or semiconductor gas sensors. These have a lower detection limit of about 1 ppm, they are affected by ambient humidity, their power consumption is rather high, and they require frequent recalibrations, especially when integrated in portable equipment. Electrochemical sensors employing a 3-electrode configuration and liquid electrolyte can be a good, inexpensive approach for detecting hydrogen sulfide in air at concentrations up to thousands of ppm with ppm resolution [8]. The typical cost of an electrochemical hydrogen sulfide sensor is about 30 US dollars. Selectivity can be achieved by the proper selection of the materials’ integrating filter, hydrophobic membrane, working electrode and electrolyte [9,10]. However, their internal architecture makes them very hard to miniaturize.
There is a strong demand for miniaturized, inexpensive sensors to be integrated in portable/wearable devices for personal protection or in distributed wireless sensor networks for environmental monitoring. In the last few years, there have been considerable research efforts devoted to the development of inorganic, organic, and hybrid nanomaterials in view of achieving hydrogen sulfide sensors with improved sensitivity, selectivity, resilience to ambient humidity, long-term stability, and low power consumption. The object of this review paper is to identify these recent efforts, summarize achievements, identify shortcomings, and discuss future research directions.
2. Metal Oxide Nanomaterials
Even though metal oxide nanomaterials are appropriate for building inexpensive and sensitive enough hydrogen sulfide sensors, they generally suffer from a lack of selectivity, are heavily affected by ambient moisture, and are power-hungry, since their optimal operating temperatures are well above room temperature. Despite these initial drawbacks, many studies have been conducted on metal oxide hydrogen sulfide sensors. The following paragraphs discuss the main results attained thus far.
Iron oxide (Fe2O3) could be anticipated to be a good candidate for detecting H2S, because the material had been widely employed as a catalyst in the oxidation of hydrogen sulfide in desulfurization processes [11]. Indeed, Zhang and co-workers [12] demonstrated that when H2S was catalytically oxidized in Fe2O3, an intense chemiluminescent (CL) signal at 400 nm could be recorded. Furthermore, the CL signal was found to change linearly with hydrogen sulfide in a wide concentration range. The CL activity of Fe2O3 in the presence of H2S was found to be at least one order of magnitude higher than that of other catalysts such as CaO, Al2O3, ZrO2, or Au-loaded WO3. In addition, Fe2O3 was very selective towards the detection of hydrogen sulfide with no cross-sensitivity to ethanol, hexane, cyclohexane, ethylene, hydrogen, o-dichlorobenzene, carbon dioxide, nitrogen dioxide, ammonia, thiophene, and sulfur dioxide. Even though it showed a CL response to dimethyl sulfur, this happened at operating temperatures higher than 360 °C, therefore, this potential cross-sensitivity could be addressed by operating Fe2O3 at 320 °C. The reason for such good performance in terms of selectivity was attributed to Fe2O3 being able to produce CL intermediates (excited SO2) upon the catalytic oxidation of H2S. The system was studied under dry conditions only and, therefore, the influence of ambient moisture in overall performance was not discussed. A similar CL behavior was observed by Liu and co-workers [13] on self-assembled SnO2 nanospheres, synthesized by a hydrothermal method. In this particular case, changes in the CL signal at 410 nm were recorded in the presence of H2S at near 200 ppb levels. However, no selectivity study was conducted.
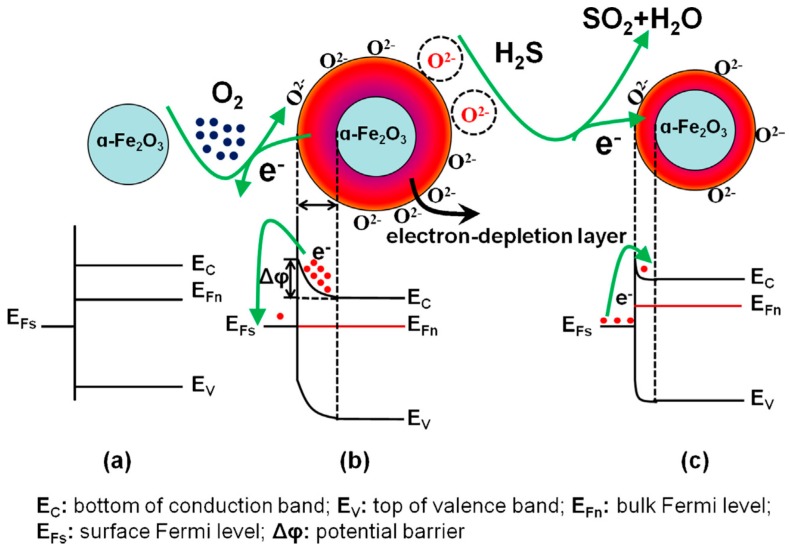
Besides its interesting chemiluminescent properties, iron oxide has also been employed as the active material in chemo-resistors for detecting hydrogen sulfide. Zheng and co-workers [14] reported the ionothermal synthesis of α-Fe2O3 nanochains and its use in resistive hydrogen sulfide sensors. Similarly, Li and co-workers [15] employed α-Fe2O3 nanoparticles for detecting H2S at the ppm level. The sensing mechanism consists of H2S altering the equilibrium concentration of oxygen adsorbates present at the surface of the iron oxide nanomaterial. At the operating temperature (i.e., near 300 °C) H2S reacts with oxygen adsorbates producing SO2 and H2O. H2S oxidation lowers the amount of ionosorbed oxygen at the surface of Fe2O3. This increases the number of charge carriers (electrons) in the conduction band of the nanomaterial (an n-type semiconductor) and lowers the width of the space charge layer that develops, upon oxygen ionosorption, at the outer shell of the Fe2O3 nanostructures. Macroscopically, this translates to a decrease in the electrical resistance of a film made of the nanomaterial. This detection is reversible because when the material is flushed with clean air, the initial concentration of ionosorbed oxygen is restored, and the baseline resistance is regained. Figure 1 illustrates this detection mechanism. Even though this nanomaterial is highly sensitive to hydrogen sulfide given its high catalytic properties for H2S oxidation, its detection mechanism implies an inherent lack of selectivity, since the presence of any reducing species able to affect the equilibrium concentration of oxygen adsorbates, would generate a confounding signal.
Figure 1.
Band diagrams and schematic images of the surface reactions of an α-Fe2O3 nanograin under different atmospheres, while kept at the optimal operating temperature (i.e., 300 °C): (a) under inert atmosphere (e.g., Ar); (b) exposed to air (c) in the presence of H2S diluted in air. Reproduced from [15], with permission from Elsevier.
In view of ameliorating the selectivity for detecting H2S, the metal loading of metal oxides and the use of spinels has been reported. For example, Wu and co-workers [16] reported the use of Pt-loaded α-Fe2O3. The presence of Pt clusters on the surface of α-Fe2O3 results in an increased concentration of oxygen adsorbates on the metal oxide. Pt adsorbs and decomposes molecular oxygen, which eventually ionosorbs on the surface of the metal oxide via a spill-over effect [17]. The increased amount of oxygen adsorbates justifies the increased response to H2S that is observed experimentally. However, this mechanism is not able to explain why there should be any selectivity improvement. Mulla and co-workers [18] employed Fe-doped SnO2. At the reported operating temperatures, iron nanoparticles are most probably oxidized and these act as sensitizers for the detection of hydrogen sulfide. However, the selectivity attained remains poor. Zhu and co-workers [19] reported the use of porous iron molybdate nanorods. A good response to hydrogen sulfide was obtained at a remarkably low operating temperature (80 °C), but no details on selectivity or ambient humidity effects were given. The use of p-type semiconductor spinels such as nickel ferrite (NiFe2O4) or Ni0.6Zn0.4Fe2O4 has been reported as well [20,21]. However, their sensitivity to H2S is significantly lower than that of the materials discussed above and their selectivity remains limited.
Besides iron-containing nanomaterials, many other metal oxides have been investigated for developing hydrogen sulfide chemo-resistors. These include WO3 [22,23,24,25], ZnO [26,27,28], In2O3 [29], CeO2 [30], and YMnO3 [31]. Pure tungsten oxide shows a response to hydrogen sulfide (Rair/Rgas = 5 at 225 °C for 20 ppm H2S) but also very high cross-sensitivity to ammonia [32] (Rair/Rgas = 3.5 at 300 °C for 100 ppm NH3) and to nitrogen dioxide [22] (given the oxidizing nature of nitrogen dioxide, the sensor resistance increases when exposed to this species; Rgas/Rair = 8 at 225 °C for 1 ppm NO2). Deng and co-workers [33] have reported a high response and a remarkable selectivity towards hydrogen sulfide of mesoporous tungsten trioxide having large pore widths, but the reason for this selectivity is not discussed and cannot be easily explained based on the size of the pores within their nanomaterial. Zinc oxide nanomaterials (nanorods and dendritic) [26,27] or ceria nanowires [30] have been shown to be responsive towards hydrogen sulfide at room temperature, an interesting result for developing ultra-low power sensors; however, they show heavy cross-sensitivity to ambient humidity and rather slow response dynamics. Indium oxide shows poor selectivity to hydrogen sulfide with important nitrogen dioxide [34] and ethanol [29] cross-sensitivity. Unlike the previously discussed materials, hexagonal YMnO3 is a p-type semiconductor. It shows moderate sensitivity to hydrogen sulfide with limited selectivity.
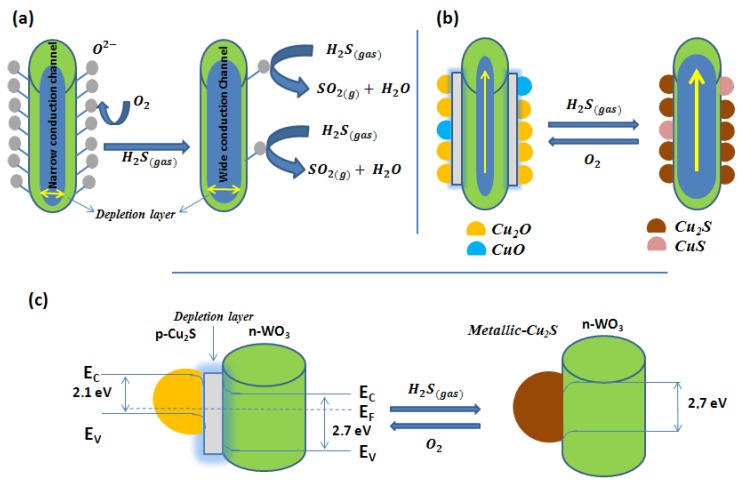
In 1994, Rao and co-workers [35] developed a H2S sensor employing CuO nanoparticles dispersed into a SnO2 matrix. The transformation of CuO, a p-type semiconductor, into CuS, which shows metallic characteristics, upon exposure to H2S is hypothesized to be the reason for the large increase observed in the conductance of the CuO-SnO2 film. Furthermore, at the sensor operating temperature (200 °C) CuS converts back to CuO when H2S is removed from the ambient conditions, which results in the reversibility of the detection mechanism. In 1998, Yamazoe and co-workers [36] and Gaskow and co-workers [37] explored further the use of CuO-SnO2 heterojunctions for the detection of H2S. Gaskov and coworkers [38] attributed the detection mechanism to the transformation of CuO into Cu2S, as observed by X-ray diffraction (XRD) analysis. The selectivity in the detection of hydrogen sulfide and the effect of ambient moisture are not reported in these initial studies. Since then, many authors have reported the use of p-type cuprous or cupric oxides onto n-type metal oxides for detecting H2S. These include CuO-SnO2 [38,39,40,41,42], Cu2O-SnO2 [43], CuO-ZnO [44,45], CuO-SnO2-ZnO [46], and Cu2O-WO3 [47] nanocomposites. Most papers show that the optimal operating temperature lies in the range from 100 °C to 400 °C, however, a few examples are given on the room-temperature detection of hydrogen sulfide [40,43]. In room-temperature operated sensors, some problems arise related to the slow response dynamics and the lack of recovery of the sensor baseline. While in most cases the response of the nanomaterials to hydrogen sulfide is only discussed, some authors properly address selectivity issues by studying the sensor response to possible interfering species [41,47], including the effect of ambient humidity [48]. According to these results, there seems to be an optimum in the amount of copper oxide loading, which lies between 0.5% and 3%, for maximizing selectivity towards H2S and minimizing cross-sensitivity to changes in the level of the ambient moisture. In many cases, the response towards H2S is found to be, at least, over 10-fold higher than that observed for the other species tested (e.g., CH4, CO, NH3, H2, C6H6, or NO2). Figure 2 illustrates the gas sensing mechanism.
Figure 2.
H2S gas sensing mechanism of (a) pure WO3 nanowires, (b) Cu2O/CuO nanoparticle-functionalized WO3 nanowires, and (c) an example of the evolution of the Cu2O nanoparticle/WO3 nanowire p-n heterojunction before and after exposure to H2S. Reproduced from [47], with permission from the American Chemical Society.
As shown in Figure 2a, when pure WO3 nanowires are exposed to air, oxygen molecules can adsorb on their surface and form chemisorbed oxygen species. Oxygen adsorbates lead to the formation of an electron depletion layer by trapping electrons via the conduction band of the n-type tungsten oxide and make the material highly resistive. When exposed to H2S, the chemisorbed oxygen species react with the reducing gas molecules producing H2O and SO2, while the electrons, originally trapped at oxygen adsorbates, are released. This results in a decrease in the resistance of the nanowires. This mechanism, which is equivalent to the one illustrated in Figure 1, is not selective, since any reducing gas could, in principle, alter the concentration of oxygen adsorbates and, therefore, would be detected. In contrast, copper oxide decorated WO3 nanowires show a different mechanism (Figure 2b,c). Copper oxide nanoparticles and WO3 nanowires are p-type and n-type, respectively. The contact between these two different materials leads to the formation of numerous p-n heterojunctions and electron depletion layers at their interface. Upon exposure to H2S, copper oxides are converted to metallic copper sulfides, by an oxygen/sulfur replacement mechanism, and the p-n heterojunctions are destroyed. Hence, a large number of electrons are released in the WO3 nanowires and a dramatic decrease in the resistance of the nanocomposite can be observed. In the recovery phase, when hydrogen sulfide is removed by a flow of clean air, the copper oxides are regenerated, the p-n heterojunctions are restored, and the material resistance returns to its original high value. This mechanism, which has been experimentally validated by performing X-ray photoelectron spectroscopy (XPS) analysis on the nanomaterials before and after their exposure to hydrogen sulfide [47], explains why copper oxide decorated metal oxide composites show an inherent selectivity to hydrogen sulfide.
Besides the use of p-n heterojunctions formed by copper oxide nanoparticles supported on an n-type metal oxide semiconductor, some authors have reported the use of pure copper oxide nanomaterials, e.g., CuO nanosheets [48] or thin films with different stoichiometry (CuO, Cu2O, or Cu4O3) [49,50]. The results reported are also very promising for the selective detection of H2S, however, the response of pure copper oxide nanomaterials seems to be affected by ambient moisture [48,50]. In contrast, some heterojunction nanomaterials have been reported to be humidity-insensitive [47]. This synergistic effect found in p-n heterojunction nanomaterials deserves further research to be better understood.
Even though the vast majority of papers in which the use of metal oxide nanomaterials is addressed for detecting hydrogen sulfide employ chemo-resistive transducing schemes, a few papers have reported optical transduction via the monitoring of changes in surface plasmon resonance (SPR). Martucci and co-workers [51] reported Au nanoparticles dispersed onto TiO2-NiO films for detecting H2S. The direct oxidation of H2S on the films resulting in SO2 is described as the sensing mechanism. The response signal is the change in absorbance intensity at a wavelength that is close to the SPR of Au nanoparticles (600 nm). Despite this, the nanomaterial seems selective to hydrogen sulfide in the presence of CO or H2, the detection limit is in the ppm range, and the signal saturates at H2S concentrations of about 5 to 10 ppm. Gupta and co-workers [52] reported positive shifts in the SPR of Cu-ZnO films with increasing concentrations of H2S (10 to 100 ppm). The mechanism of detection is not discussed, but most probably the shift observed is due to the sulfurization of Cu upon exposure to H2S. In a very similar approach, the same group reported SPR in nickel oxide doped indium tin oxide (ITO) films over silver [53], and in ZnO nanoparticles or nanorods [54,55]. Positive shifts in the SPR of these films with increasing concentrations of H2S are observed. This is attributed to the formation of NiS or ZnS upon exposure to H2S. No significant cross-sensitivity towards ammonia, chlorine, or carbon monoxide is reported. A saturation of the SPR response at about 80 to 100 ppm is observed.
3. Functionalized Carbon Nanomaterials
In 2009, Mhaisalkar and co-workers [56] described the use of Ag decorated single walled carbon nanotubes (SWCNT) for detecting H2S at room temperature in a background of nitrogen. These resistive sensors show an irreversible response towards H2S, due to the formation of Ag2S. Furthermore, the sensor is not selective, since it shows cross-sensitivity to nitric oxide and carbon monoxide. In 2010, Deshusses and co-workers [57] employed Au-decorated SWCNTs for detecting H2S at room temperature in air. The detection of H2S at a concentration as low as 20 ppb was shown. Operated as resistive films on a back gate configuration, the affinity between gold and sulfur and the fact that the work function of Au changes upon the chemisorption of H2S is hypothesized as the sensing mechanism. When proper back gate voltages are applied, the sensor signal is reversible, which is attributed to mild heating of the Au-SWCNT mat. However, it is well-known that Au-decorated carbon nanotubes are very sensitive to other gaseous species such as NO2 [58] and no selectivity studies were performed. More recently, Yoon and co-workers [59] reported the use of a Co3O4-SWCNT composite for detecting H2S at 250 °C. The role of the small amount of carbon nanotubes within the nanocomposite is to render the cobalt oxide nanoparticles more defective and, thus, more sensitive to hydrogen sulfide. A selectivity study was performed in which small cross-sensitivity to ammonia, methane, and hydrogen was reported. Besides the use of carbon nanomaterials in resistive H2S sensors, Cu-decorated SWCNTs have been employed together with gravimetric transducers [60]. The sensor is responsive to H2S at ppm levels and no significant cross-sensitivity to hydrogen, ethanol or acetone is reported. The effect of ambient moisture on sensor response is also discussed. A 40% relative humidity (R.H.) level completely destroys room temperature sensitivity. To recover the response signal in the presence of humidity, the device has to be operated at temperatures well above 100 °C.
Pal and co-workers [61] reported the potential of using a composite consisting of a tin oxide thin film coated with diamond like carbons (DLC) in which Cu nanoparticles had been embedded. It was observed that soon after exposure to hydrogen sulfide (about 3 s), the characteristic surface plasmon resonance peak, located at 650 nm, disappeared. This was attributed, via XRD and Raman studies to the formation of CuxS during the exposure to H2S. These results are very preliminary, since a rather high concentration of hydrogen sulfide was tested (i.e., 1000 ppm) and the dynamic range of the response, reversibility, selectivity, and stability of the detector were not investigated.
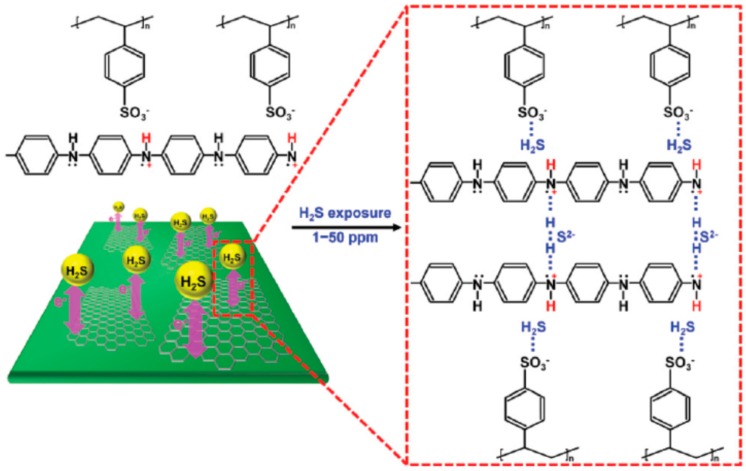
In the last few years, the use of graphene and graphene-related materials and composites has gained attention for the detection of hydrogen sulfide. Chen and co-workers [62] described the use of Cu2O onto functionalized graphene sheets (FGS). FGS acts as a molecular template for the controlled nucleation of Cu2O nanoparticles, the size of which is controlled via the C/O ratio in the FGS. Upon adsorption of hydrogen sulfide, Cu2O nanoparticles inject electrons onto the p-type FGS, which results in an increase in the resistance of the nanocomposite film. The detection of hydrogen sulfide down to 5 ppb, and operating the sensors at room temperature is demonstrated. Selectivity is also described, since small responses to hydrogen, ammonia, ethanol and methane were reported, but no information is given on cross-sensitivity to ambient moisture or nitrogen dioxide. In a similar approach, Jahangiri and co-workers [63] have reported MoO3 on reduced graphene oxide (MoO3/rGO). However, the optimal operating temperature was 160 °C and the limit of detection for H2S was rather high, near 50 ppm. Jang and co-workers [64], reported the use of a conducting polymer/graphene nanocomposite, namely poly(4-styrenesulfonic acid)-doped polyaniline/graphene (PSS-doped PANI/graphene) operated at room temperature. PSS was used as a doping and a binding agent for the polymerization of aniline monomers. The PSS allowed the dispersion of reduced graphene sheets through electrostatic repulsion. PSS-doped PANI/graphene composites containing 30 wt% graphene showed the highest response to hydrogen sulfide at ppm levels. As sensing mechanisms, the formation of additional N–H bonds that appear in the PSS-doped PANI when in the presence of hydrogen sulfide is suggested. This would result in the experimentally observed decrease in the resistance of the PSS-doped PANI structure. Figure 3 illustrates this mechanism. The dispersed graphene sheets allow higher electric currents to flow through the sensor electrode, which significantly increases the response and lowers the detection limit for hydrogen sulfide. This composite, however, shows an important cross-sensitivity to ammonia.
Figure 3.
Possible mechanism for molecular interactions between the PSS-doped PANI/graphene nanocomposites and H2S gas molecules. Reproduced from [64], with permission from the Royal Society of Chemistry.
Recently, the use of amide or amine functionalized graphene oxide has been reported for the detection of hydrogen sulfide at room temperature [65,66]. However, the response is rather weak and subject to strong cross-sensitivity issues.
4. Other Nanomaterials and Transduction Approaches
In 2013, Xie and co-workers [67] reported organic thin film transistors employing silicon dioxide as a dielectric layer and copper phthalocyanines as gas sensitive materials for detecting hydrogen sulfide. The detection of hydrogen sulfide was demonstrated at room temperature in the hundreds of ppm range, with some reversibility problems. These sensors suffered from important cross-sensitivity to SO2 and the effect of ambient moisture was not reported. Furthermore, Cu-phthalocyanine sensors for detecting ethanol, nitrogen dioxide, water vapor, or volatile organic compounds have been reported [68,69], so important selectivity issues can be anticipated.
Liu and co-workers [70] reported organic-inorganic composites based on polythiophene (PT), a p-type semiconductor, and WO3 (n-type) for detecting H2S at low operating temperatures (70 °C). The changes observed in the electrical resistance of the film are attributed to the decrease in the width of the space charge region that develops at the PT-WO3 interface, when exposed to hydrogen sulfide. These sensors show low cross-sensitivity to methanol, ethanol, propanol, and ammonia, however, no data on humidity interference is shown.
Recently, Tang and co-workers [71,72] have shown that colloidal PbS quantum dots (QDs) show remarkable sensitivity and selectivity to H2S when operated at 135 °C. A 4000-fold decrease in resistance is reported when the sensor is exposed to 50 ppm of hydrogen sulfide. The originally oleic-acid caped PbS QDs were treated with Pb(NO3)2 for ligand exchange. The gas sensing mechanism may be twofold: the interaction with hydrogen sulfide causes the removal of adsorbed oxygen on the surface of the QDs, which change from p-type to n-type. Additionally, H2S adsorption creates donor states near the conduction band. As a result of these two combined effects, a dramatic change in resistance is observed. Furthermore, a remarkable selectivity towards H2S is shown, with little cross-sensitivity to SO2, NO2, and NH3. Once more, measurements were performed under dry conditions only and no information on ambient moisture effects was shown. This new approach seems very promising, yet the results reported so far are somewhat preliminary. More efforts are needed to better understand and model the sensing mechanisms for the technique to increase in maturity.
Metal-organic frameworks (MOFs) are built from clusters of metal ions and multidentate organic ligands. MOFs offer a wide spectrum of structures, pore sizes, and metal catalytic sites, together with high specific area to interact with gases and good thermal stability. In the last few years, MOFs have attracted interest for designing gas sensors, including H2S sensors [73]. In this paper, different MOFs were assessed for the detection of H2S at ppm levels, and Zn3(BTC)2·12H2O or ZIF-8 showed a remarkably intense and selective cataluminescence (CL) response at 250 °C. According to the results, the nature of the metal sites is more important than the ligands within the MOFs for producing a CL signal, even though the latter modulate the intensity of this response. Extraordinary fast response and recovery times below 1 s and 5 s, respectively, have been reported. Furthermore, MOFs could also be employed as functional coatings onto metal oxide nanomaterials, as reported in [74]. Acting as molecular sieves, MOFs could be of help for improving selectivity.
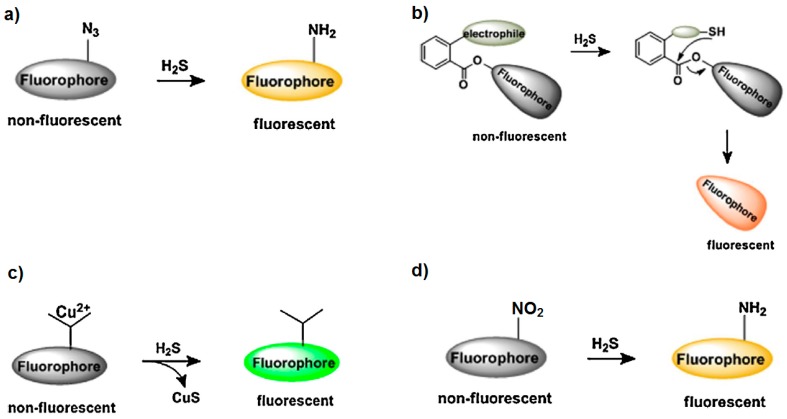
The selective detection of H2S employing fluorescent probes has also been reported. Fluorescent probes for detecting hydrogen sulfide employ four different reactions to produce an easily readable, turn-on fluorescence [75]. These reactions consist of the selective reduction of azides into amines by H2S; the nucleophilic addition of H2S to the probe which results in a cyclization process that generates a fluorescent molecule; copper displacement in which H2S mediates precipitation of CuS to induce turn-on fluorescence; and nitro to amine reduction, in which H2S selectively reduces the nitro group into amine, producing turn-on of the fluorescence. Figure 4 gives a schematic overview of these four different approaches. Although this approach could be used to assess H2S in the environment, fluorescent probes have been specifically developed for selectively detecting H2S emitted by cells in living organisms as a biomarker of different diseases including cancer or Alzheimer’s disease [76,77].
Figure 4.
Reactions for the selective detection of H2S employing fluorescent probes. Reduction of azides into amines by H2S (a), nucleophilic addition of H2S to the probe resulting in a cyclization process that generates a fluorescent molecule (b), H2S mediates the precipitation of CuS (c), nitro to amine reduction (d). Adapted from [75], with permission from Elsevier.
5. Discussion
Considering the transducing schemes employed for detecting H2S, a significant majority of what has been reported discusses chemo-resistors. However, other approaches comprise chemo-field effect transistors (chemFET), gravimetric surface acoustic wave (SAW) or optical read-out via chemo-luminescence, monitoring of shifts in SPR, lossy mode resonance or absorbance changes in optical fiber sensors, and fluorescence (for detecting H2S in liquids). The sensitive materials reported include inorganic (metal oxides), carbon nanomaterials (carbon nanotubes, graphene-related, and diamond like, organic materials (phthalocyanines, conducting polymers) and organic-inorganic hybrid nanomaterials. Table 1 summarizes the properties and the main performance characteristics of the different nanomaterials and transducing schemes that have been reported in the detection of hydrogen sulfide.
Table 1.
Summary of the main properties and performance characteristics of the different nanomaterials employed in the detection of H2S gas.
| Nanomaterial | Transduction Scheme | Operating Temperature | Response to H2S | Range and LOD | Response Time | Recovery Time | Selectivity (Not Affected by, Unless Otherwise Indicated) | Reference |
|---|---|---|---|---|---|---|---|---|
| α-Fe2O3 film | Chemi luminescence | 320 °C | 1827 (100 ppm) 1 | 8–2000 ppm/3 ppm | 15 s | 120 s | Ethanol, hexane, cyclohexane, ethylene, hydrogen, o-dichlorobenzene, carbon dioxide, nitrogen dioxide, ammonia, thiophene, sulfur dioxide, dimethyl sulfur. Effect of ambient moisture not available. | [12] |
| SnO2 nanospheres | Chemi luminescence | 160 °C | 2000 (5 ppm) 1 | NA 5 | 4 s | 20 s | Carbon monoxide, hydrogen, nitrogen dioxide. Effect of ambient moisture not available. | [13] |
| α-Fe2O3 nanochains | Chemo resistor | 285 °C | 20 (100 ppm) 2 | 1–100 ppm/1 ppm | 8.6 s | 66 s | NA 5 | [14] |
| α-Fe2O3 nanoparticles | Chemo resistor | 300 °C | 5 (10 ppm) 2 | 0.05–100 ppm/50 ppb | 30 s | 5 s | NA 5 | [15] |
| Pt-doped α-Fe2O3 film | Chemo resistor | 160 °C | 330 (100 ppm) 2 | 10–1000 ppm/units of ppm | >1 min | >1 min | Ethane, carbon monoxide, significant cross-sensitivity to ethanol and ammonia. Effect of ambient moisture not available. | [16] |
| Fe-doped SnO2 | Chemo resistor | 150 °C | 90 (100 ppm) 2 | 10–250 ppm/10 ppm | Few s | 100 s | Carbon monoxide, liquefied petroleum gas, significant cross-sensitivity to ethanol. Effect of ambient moisture not available. | [18] |
| Fe2(MoO4)3 nanorods | Chemo resistor | 80 °C | 18 (50 ppm) 2 | 1–50 ppm/1 ppm | 30 s | 150 s | NA 5 | [19] |
| NiFe2O4 | Chemo resistor | 300 °C | 35.8 (5 ppm) 2 | 5–200 ppm/1 ppm | 15 s | 35 s | Liquefied petroleum gas, methane, carbon monoxide, butane, significant cross-sensitivity to hydrogen. Effect of ambient moisture not available. | [20] |
| Ni0.6Zn0.4Fe2O4 | Chemo resistor | 225 °C | 0.8 (50 ppm) 3 | NA 5 | 10 s | 95 s | Significant cross-sensitivity to ethanol, liquefied petroleum gas, ammonia. Effect of ambient moisture not available. | [21] |
| Pure and metal loaded WO3 | Chemo resistor | 200 °C to 350 °C | 25–190 (10 ppm) 2 | 0.2–200 ppm/200 ppb | 1 s | 11 min | Significant cross-sensitivity to ammonia, nitrogen dioxide and ambient moisture. | [22,23,24,25] |
| Mesoporous WO3 | Chemo resistor | 250 °C | 325 (100 ppm) 2 | 0.25–200 ppm/250 ppb | 2 s | 38 s | Hydrogen, benzene, with some cross-sensitivity to methanol, ethanol, acetone, ammonia, and acetaldehyde. Effect of ambient moisture not available. | [33] |
| Pure or In doped ZnO | Chemo resistor | RT 4 to 250 °C | 17 to 90 (100 ppm) 2 | 0.5 to 100 ppm/500 ppb | >20 min (RT); 2 s (250 °C) | >20 min (RT); 4 min (250 °C) | High cross-sensitivity to ammonia. Effect of ambient moisture not available. | [26,27,28] |
| ZnO nanoparticles | Shift of SPR peak | RT 4 | 0.71 nm/ppm 6 | 10 to 100 ppm/NA 5 | 1 min | 1 min | Hydrogen, methane, ammonia, chlorine. Effect of ambient moisture not available. | [54] |
| ZnO nanorods | Lossy mode resonance | RT 4 | 0.8 nm/ppm 6 | 10 to 100 ppm/NA 5 | NA 5 | NA 5 | Hydrogen, methane, ammonia, chlorine. Effect of ambient moisture not available. Response saturates at 60 ppm of H2S. | [55] |
| In2O3 | Chemo resistor | 270 °C | 120 (50 ppm) 2 | NA 5 | NA 5 | NA 5 | Hydrogen, ammonia, toluene, benzene, carbon monoxide, methane. Significant cross-sensitivity to liquefied petroleum gas, formaldehyde, and ethanol. Important cross-sensitivity to nitrogen dioxide. Effect of ambient moisture not available. | [29,34] |
| CeO2 | Chemo resistor | RT 4 | 2 (100 ppm) 2 | 0.1 to 100 ppm/100 ppb | 20 s | 200 s | Hydrogen, with some cross-sensitivity to ethanol and ammonia. Effect of ambient moisture not available. | [30] |
| YMnO3 | Chemo resistor | 100 °C | 90 (500 ppm) 2 | 20 to 100 ppm/NA 5 | 6 s | 6 s | Significant cross-sensitivity to liquefied petroleum gas, carbon monoxide, and hydrogen. Effect of ambient moisture not available. Response saturates for H2S concentrations higher than 100 ppm. | [31] |
| CuO-SnO2 | Chemo resistor | 200 °C | 0.88 (100 ppm) 3 | 100 to 500 ppm/NA 5 | 60 s | 40 s | NA 5. Response saturates for H2S concentrations higher than 300 ppm. | [35] |
| CuO-SnO2 | Chemo resistor | 300 °C | 88 (2 ppm) 2 | 20 to 1000 ppb/20 ppb | 10 min | 10 min | NA 5 | [36] |
| CuO-SnO2 | Chemo resistor | 160 °C | 250 (100 ppm) 2 | 25 to 300 ppm/NA 5 | 10 min | NA 5 | NA 5 | [37] |
| CuO-SnO2 | Chemo resistor | 140 °C | 106 (10 ppm) 2 | 10 to 160 ppm/NA 5 | 2 min | >20 min | NA 5 | [38] |
| CuO-SnO2 | Chemo resistor | 150 °C | 2000 (20 ppm) 2 | NA 5 | 14 s | 21 s | NA 5 | [39] |
| CuO-SnO2 nanoribbons | Chemo resistor | RT 4 | 1.8 × 104 (3 ppm) 2 | NA 5 | 15 s | >20 min | NA 5 | [40] |
| CuO-SnO2 | Chemo resistor | 150 °C | 0.81 (1000 ppm) 3 | 200–2500 ppm/tens of ppm | 53 s | 83 s | Hydrogen, carbon monoxide, liquefied petroleum gas, sulfur dioxide. Effect of ambient moisture not available. Response saturates for H2S concentrations higher than 1500 ppm. | [41] |
| CuO-SnO2 coral-like | Chemo resistor | 100 °C | 4173 (10 ppm) 2 | 0.1–10 ppm/20 ppb | 10 s | >30 min | Ethanol, formaldehyde. Effect of ambient moisture not available. | [42] |
| Cu2O-SnO2 | Chemo resistor | RT 4 | 0.6 (100 ppm) 3 | 5–150 ppm/1 ppm | >1 min | >1 min | Toluene. Significant cross-sensitivity to hydrogen liquefied petroleum gas, nitric oxide, ammonia. Effect of ambient moisture not available. | [43] |
| CuO-ZnO | Chemo resistor | 225 °C | 380 (10 ppm) 2 | 0.1–20 ppm/100 ppb | 10 s | 200 s | Ethanol, acetone, hydrogen, nitrogen dioxide, sulfur dioxide, methane, acetaldehyde. Effect of ambient moisture not available. | [44] |
| CuO-ZnO | Chemo resistor | 200 °C | 83.5 (5 ppm) 2 | 5–100 ppm/1 ppm | 572 s | 65 s | Carbon monoxide, ammonia, hydrogen, methane. Effect of ambient moisture not available. | [45] |
| Cu-ZnO | Shift of SPR peak | RT 4 | 0.2 nm/ppm 6 | 10–100 ppm/NA 5 | 1 min | 1 min | NA 5 | [52] |
| Cu-SnO2-ZnO | Chemo resistor | 150 °C | 6.4 × 104 * | NA 5 | NA 5 | NA 5 | Liquefied petroleum gas, carbon dioxide, nitrogen oxides, methane. Significant cross-sensitivity to carbon monoxide. Effect of ambient moisture not available. | [46] |
| Copper oxides-WO3 | Chemo resistor | 390 °C | 26 (5 ppm) 2 | 0.3–5 ppm/100 ppb | 2 s | 684 s | Hydrogen, carbon monoxide, ammonia, benzene, nitrogen dioxide. Resilient to changes in the background humidity. | [47] |
| CuO nanosheets | Chemo resistor | 100 °C | 320 (1 ppm) 2 | 30–1200 ppb/20 ppb | 10 s | 10 s | Ammonia, carbon monoxide, nitrogen oxides, hydrogen. Strong cross-sensitivity to ambient moisture. | [48] |
| CuO | Chemo resistor | Switching 150–450 °C | 1000 (5 ppm) 2 | NA 5 | <1 min | <1 min | Sulfur dioxide, nitrogen dioxide, ammonia. Slightly affected by changes in ambient moisture. | [50] |
| Au-TiO2-NiO | Absorbance change | 350 °C | 0.97 (100 ppm) 7 | 2–100 ppm/NA 5 | 1 min | 7 min | Carbon monoxide, hydrogen. Effect of ambient moisture not available. Response signal saturates for H2S concentrations higher than 10 ppm. | [51] |
| Ag-NiO-ITO | Shift of SPR peak | RT 4 | 1 nm/ppm 6 | 10–100 ppm/NA 5 | NA 5 | NA 5 | Hydrogen, ammonia, chlorine, carbon monoxide. Effect of ambient moisture not available. | [53] |
| Ag-SWCNT | Chemo resistor | RT 4 | NA 5 | NA 5 | NA 5 | NA 5 | Cross-sensitivity to ammonia and nitric oxide. Effect of ambient moisture not available. Unstable response. Lack of baseline recovery. | [56] |
| Au-SWCNT | Chemo resistor | RT 4 | 0.23 (1 ppm) 3 | 20–1000 ppb/20 ppb | 5 min | >20 min | NA 5. Response saturates at 250 ppb of H2S. | [57] |
| Co3O4–SWCNT | Chemo resistor | 250 °C | 5 (100 ppm) 3 | 10–150 ppm/units of ppm | 2 min | 10 min | Ammonia, methane, hydrogen. Effect of ambient moisture not available. | [59] |
| Cu-MWCNT | SAW | 150 °C | 240 kHz (100 ppm) 8 | 5–150 ppm/units of ppm | 7 s | 9 s | Hydrogen, ethanol, acetone. Ambient moisture affects the response (20% decrease in response at 150 °C). | [60] |
| Cu-Diamond Like Carbon | Shift of SPR peak | RT 4 | NA 5 | NA 5 | 1 min | NA 5 | NA 5 | [61] |
| Cu2O-Graphene | Chemo resistor | RT 4 | 0.35 (100 ppb) 3 | 5–100 ppb/1 ppb | 2 min | 10 min | Hydrogen, methane, ammonia. Significant cross-sensitivity to ammonia. Effect of ambient moisture not available. | [62] |
| MoO3–rGO | Chemo resistor | 160 °C | 4120 (50 ppm) 2 | 50–500 ppm/50 ppm | 1 min | 2 min | Ethanol, nitric oxide, carbon monoxide. Effect of ambient moisture not available. | [63] |
| PSS-doped PANI/graphene | Chemo resistor | RT 4 | 0.79 (50 ppm) 3 | 1–50 ppm/1 ppm | 5 min | >20 min | Ethanol. Significant cross-sensitivity to ammonia. Effect of ambient moisture not available. | [64] |
| DDA-GO or EDA-GO or AMIDE-GO | Chemo resistor | RT 4 | 160 (50 ppm) 2 | 50–500 ppm/50 ppm | 1 min | 3 min | Carbon monoxide, nitric oxide. Significant cross-sensitivity to ethanol. Effect of ambient moisture not available. | [65,66] |
| Cu-Phthalo cynanine | ChemFET | RT 4 | 0.605 (200 ppm) 9 | 100–500 ppm/NA 3 | 89 s | 290 s | Methane, hydrogen, carbon monoxide. Significant cross-sensitivity to ammonia. Effect of ambient moisture not available. Poor recovery of the baseline. | [67] |
| Polytiophene-WO3 | Chemo resistor | 70 °C | 12 (100 ppm) 2 | 5–200 ppm/3 ppm | 10 s | 5 s | Methanol, ethanol, acetone, ammonia. Effect of ambient moisture not available. | [70] |
| Colloidal PbS QDs | Chemo resistor | 135 °C | 4218 (50 ppm) 2 | 5–100 ppm/6 ppb | 23 s | 171 s | Sulfur dioxide, nitrogen dioxide, ammonia. Effect of ambient moisture not available. | [71,72] |
| MOFs | Chemi luminescence | 250 °C | 7500 (16 ppm) 1 | 6–16 ppm/1 ppm | 1 s | 5 s | Methanol, ethanol, propanol, butanol, isobutanol, acetone, formaldehyde, toluene, chloroform. Effect of ambient moisture not available. | [73] |
1 Chemoluminescence signal; 2 Rair/Rgas; 3 (Rair − Rgas)/Rair; 4 RT: Room temperature; 5 NA: Not available; 6 Shift in the SPR frequency; 7 Absgas/Absair; 8 Frequency shift; 9 (Igas − Iair)/Iair; * Not clear how this sensitivity value is obtained.
Considering the results summarized in Table 1, the most promising approaches are as follows.
Employing α-Fe2O3 at temperatures near 300 °C, which presents strong chemiluminescence upon interaction with hydrogen sulfide at 400 nm. Very remarkable selectivity is achieved because α-Fe2O3 is able to produce CL intermediates (excited SO2) upon the catalytic oxidation of H2S. Furthermore, this mechanism enables measuring H2S in a wide concentration range (up to thousands of ppm) and tens of seconds and minutes have been reported as response and recovery times, respectively [12]. The process for obtaining α-Fe2O3 is standard and can be easily scaled up for mass production. It involves the precipitation of ferric hydroxide employing an aqueous solution of FeCl3 with ammonia, which is washed in water and further oxidized by annealing at 600 °C for 3 h. Despite all these interesting characteristics, studying the effect of ambient moisture on the sensor response and the long-term stability of the material would be necessary to fully validate this approach. In addition, monitoring CL at 400 nm involves using a chemiluminescence analyzer and optical filters, which are not easy to miniaturize and may be rather expensive.
Heterojunction nanomaterials that comprise p-type CuO or Cu2O and an n-type metal oxide such as SnO2, ZnO, or WO3. The detection mechanism consists of the strong electronic sensitization brought about by the copper oxides, which sulfurize to CuS or Cu2S upon exposure to H2S. In contrast to copper oxides, which are p-type semiconductors, copper sulfides are metallic and, therefore, exposure to H2S results in a dramatic increase in conductivity of the p-n nanomaterial. Furthermore, this sulfurization process is reversible, i.e., copper oxides are regenerated upon cleaning with air, which makes this approach very interesting for the chemoresistive sensing of H2S. Employing this approach, the detection of hydrogen sulfide in a wide concentration range has been reported (200 ppb to 1500 ppm) with a lower detection limit of about 20 ppb [35,36,37,38,39,40,41,42,43,44,45,46,47,52]. The best response and recovery times reported are units and tens of seconds, respectively. The optimal operating temperature lies between 140 °C and 400 °C, however, Gupta and co-workers [55] reported a remarkable response towards H2S despite sensors being operated at room temperature. Even though metal oxides are known to be poorly selective, the detection of hydrogen sulfide is a special niche in which copper oxide based nanomaterials can reach remarkable selectivity, due to the specific detection mechanism discussed above. Overall, these sensors do not present significant cross-sensitivity to many species such as hydrogen, carbon monoxide, liquefied petroleum gas, sulfur dioxide, ethanol, acetaldehyde, formaldehyde, toluene, benzene, ammonia, nitric oxide, nitrogen dioxide, methane, or acetone. While the effect of ambient moisture on responsiveness to hydrogen sulfide is almost never reported, Llobet and co-workers show in [47] that changes in background humidity only mildly affect H2S response. The processes employed for obtaining these nanomaterials are very different and eventually lead to strong dispersion in the performance characteristics of the sensors. Hydrothermal synthesis, precipitation formed salt precursors, and subsequent annealing or different types of chemical vapor deposition have been reported as synthesis methods, to name a few. The reported response intensities towards hydrogen sulfide in copper oxide supported on n-type metal oxides lie in a very wide range. The different synthesis methods employed result in materials with very different microstructures, namely, crystallite size, film porosity, and thickness. Considering what has been summarized in Table 1, higher response intensities are reported for thin films consisting of highly-porous materials with small crystallite sizes [37,38,39] or porous nanomaterials (nanorods, nanowires, or nanoribbons) having small diameters and supporting homogeneously-distributed, low-diameter copper oxide nanoparticles [40,42,44]. In principle, those synthesis methods that enable the procurement of nanomaterials with well controlled crystallinity (e.g., single crystalline) and nanodimensions (e.g., particles, wires, rods) would help increase the reproducibility of results and long term stability.
Quantum dots that allow a chemo-resistive read-out of H2S concentrations are also attractive. Employing PbS QDs, the detection of hydrogen sulfide in a concentration range that lies between units and hundreds of ppm has been reported with a lower detection limit of about 2 ppb [71,72]. The best response and recovery times reported are about twenty and two hundred seconds, respectively, and the optimal operating temperature is 135 °C. Even though the effect of ambient humidity has not been checked, cross-sensitivity to sulfur dioxide, nitrogen dioxide, or ammonia is low. H2S inducing p-to-n transition of PbS QDs has been suggested as the sensing mechanism. The fact that colloidal QDs are solution-processed nanomaterials is very attractive for keeping the fabrication costs of these sensors very low. However, further research would be necessary to fully validate this approach.
Some applications for hydrogen sulfide detection would require sensors to operate under harsh conditions such as low oxygen content or high temperature environments. This can be the case of H2S detection in petrochemical or power plants and in oil drilling facilities. Although none of the reviewed papers specifically discuss performance under harsh conditions, it can be anticipated that those sensors relying on the sulfurization of copper oxides upon exposure to hydrogen sulfide are good candidates for detecting this species with low oxygen content. This is because in this approach, the main sensing mechanism does not imply a change in the amount of the surface oxygen species. Indeed, some authors have already reported stable gas sensing responses employing metal oxides at trace level oxygen concentrations [23,78]. Recently, a few authors have reported SiC based materials for detecting gases at high temperatures [79,80,81], however, selective H2S detection at high temperatures has not been reported so far.
The results achieved by employing carbon nanomaterials show that these have potential for the detection of hydrogen sulfide. In most cases, fair responsiveness to H2S is reported at room temperature, which is of interest for wearable, battery operated detectors [56,57,61,62,64,65,66]. While most papers show hydrogen sulfide responses at ppm levels, Chen and co-workers [62] report a limit of detection of 1 ppb, with response and recovery times of a few minutes and tens of minutes, respectively. However, sensors employing carbon nanomaterials still present important drawbacks including lack of baseline recovery, poor stability, and important cross-sensitivity (to ambient moisture, ammonia, or nitrogen dioxide). Furthermore, in graphene based materials, many of the currently employed synthesis or preparation methods are not industrially scalable and, therefore, are not suitable for the mass production of H2S sensors.
6. Conclusions and Outlook
The last few years have seen a systematic increase in the demand for nanomaterials to be integrated in inexpensive, widespread gas detectors or gas sensing networks with superior performance and low power consumption. Here we have reviewed the advances for detecting hydrogen sulfide in the environment and identified the materials and methods that have reached maturity or that look more promising. In particular, it has been shown that iron oxide can be used for selectively detecting H2S, thanks to the chemiluminescence resulting from the catalytic oxidation of hydrogen sulfide onto this oxide. In addition, hybrid metal oxides containing cupric or cuprous oxides are excellent candidates for the selective detection of hydrogen sulfide, thanks to the formation of copper sulfides upon interaction with H2S. This constitutes one of the very few examples in which the selective detection of a target gas seems possible by employing uncomplicated, metal oxide chemo resistors. By further exploiting nanotechnology, it can be expected that more sensitive (thanks to higher specific surface area) and more stable (e.g., via better control of crystallinity and surface defects) metal oxides will be made available, which will operate at lower temperatures (even at room temperature). Additionally, the need of sensors that are able to reliably detect hydrogen sulfide under harsh conditions (e.g., low oxygen background or high temperatures) is likely to fuel the quest for metal oxides and silicon carbide hybrid materials. Organic-inorganic hybrid nanomaterials, especially those employing carbon nanomaterials, show potential for detecting hydrogen sulfide, but substantial research efforts are needed for these to reach a stage of maturity to be considered as valid alternatives. While one of their main advantages is the room-temperature responsiveness to H2S, efforts should be directed towards increasing selectivity, ameliorating stability, and reproducibility. Concerning the last issue, there is still a need for developing synthesis and functionalization techniques, especially in graphene, graphene oxide, and reduced graphene oxide materials, that enable the mass production of nanomaterials with highly reproducible properties. Finally, colloidal quantum dots also seem to be an interesting alternative for developing sensitive and remarkably selective chemo resistors for detecting hydrogen sulfide at low operating temperatures. These are solution-processed nanomaterials and, therefore, are suitable for the mass production of a new generation of inexpensive sensors on flexible substrates. However, the results reported remain preliminary and the use of lead sulfur QDs may represent a threat to the environment, therefore research on more environmentally-friendly materials should be considered to further develop this approach.
Acknowledgments
This work is funded in part by MINECO and ERDF under project No. TEC2015-71663-R; by the French government research program “Investissements d’avenir” through the IMobS3 Laboratory of Excellence (ANR-10-LABX-16-01), by the European Union through the Regional Competitiveness and Employment program 2014–2020 (ERDF—Auvergne region) and by the Auvergne region. E.L. is supported by the Catalan Institution for Research and Advanced Studies under the 2012 Edition of the ICREA Academia Award.
Author Contributions
E.L. conducted the literature search, coordinated the selection of key papers, and contributed to the writing of the paper. J.B. participated in the selection of key papers and took part in the writing of the paper. A.P., A.N., and C.V. contributed to the writing of the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Occupational Safety and Health Administration, USA, OSHA Standards: Hydrogen sulfide exposure. [(accessed on 23 January 2017)]; Available online: https://www.osha.gov/SLTC/hydrogensulfide/standards.html.
- 2.Kim K.H., Choi Y.J., Jeon E.C., Sunwoo Y. Characterization of malodorous sulfur compounds in landfill gas. Atmos. Environ. 2005;39:1103–1112. doi: 10.1016/j.atmosenv.2004.09.083. [DOI] [Google Scholar]
- 3.Kim K.H., Jeon E.C., Choi Y.J., Koo Y.S. The emission characteristics and the related malodor intensities of gaseous reduced sulfur compounds (RSC) in a large industrial complex. Atmos. Environ. 2006;40:4478–4490. doi: 10.1016/j.atmosenv.2006.04.026. [DOI] [Google Scholar]
- 4.Zhang L., De Schryver P., De Gusseme B., De Muynck W., Boon N., Verstraete W. Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: A review. Water Res. 2008;42:1–12. doi: 10.1016/j.watres.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) WHO; Geneva, Switzerland: 2003. [(accessed on 23 January 2017)]. Concise International Chemical Assessment Document 53, Hydrogen sulfide: Human health aspects. Available online: http://www.who.int/ipcs/publications/cicad/en/cicad53.pdf. [Google Scholar]
- 6.US Environmental Protection Agency (US EPA) Washington, DC, USA: 2003. [(accessed on 23 January 2017)]. Toxicological Review of Hydrogen Sulfide. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1006BZD.TXT. [Google Scholar]
- 7.Shanthi K., Balasubramanian N. A Simple Spectrophotometric Method for the Determination of Hydrogen Sulfide Based on Schiff’s Reaction. Microchem. J. 1996;53:168–174. doi: 10.1006/mchj.1996.0024. [DOI] [Google Scholar]
- 8.Pandey S.K., Kim K.H., Tang K.T. A review of sensor-based methods for monitoring hydrogen sulfide. Trends Anal. Chem. 2012;32:87–99. doi: 10.1016/j.trac.2011.08.008. [DOI] [Google Scholar]
- 9.Membrapor, AG, Switzerland: Electrochemical gas sensors for detecting H2S. [(accessed on 23 January 2017)]. Available online: http://www.membrapor.ch/
- 10.Alphasense, Ltd, United Kingdom.: Alphasense hydrogen sulphide gas sensors. [(accessed on 23 January 2017)]. Available online: http://www.alphasense.com/index.php/products/hydrogen-sulfide-safety/
- 11.Davydow A., Chuang K.T., Sanger A.R. Mechanism of H2S oxidation by ferric oxide and hydroxide surfaces. J. Phys. Chem. B. 1998;102:4745–4752. doi: 10.1021/jp980361p. [DOI] [Google Scholar]
- 12.Zhang Z., Jiang H., Xing Z., Zhang X. A highly selective chemiluminescent H2S sensor. Sens. Actuators B Chem. 2004;102:155–161. doi: 10.1016/j.snb.2004.04.015. [DOI] [Google Scholar]
- 13.Miao Z., Wu Y., Zhang X., Liu Z., Han B., Ding K., An G. Large-scale production of self-assembled SnO2 nanospheres and their application in high-performance chemiluminescence sensors for hydrogen sulfide gas. J. Mater. Chem. 2007;17:1791–1796. doi: 10.1039/b617114a. [DOI] [Google Scholar]
- 14.Ma J., Mei L., Chen Y., Li Q., Wang T., Xu Z., Duan X., Zheng W. α-Fe2O3 nanochains: ammonium acetate-based ionothermal synthesis and ultrasensitive sensors for low-ppm-level H2S gas. Nanoscale. 2013;5:895–898. doi: 10.1039/C2NR33201A. [DOI] [PubMed] [Google Scholar]
- 15.Lia Z., Huang Y., Zhang S., Chen W., Kuang Z., Ao D., Liu W., Fu Y. A fast response and recovery H2S gas sensor based on α-Fe2O3 nanoparticles with ppb level detection limit. J. Hazard. Mat. 2015;300:167–174. doi: 10.1016/j.jhazmat.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Wang S., Zhao Y., Zhu B., Kong F., Wang D., Wu S., Huang W., Zhang S. H2S sensing characteristics of Pt-doped α-Fe2O3 thick film sensors. Sens. Actuators B Chem. 2007;125:79–84. doi: 10.1016/j.snb.2007.01.037. [DOI] [Google Scholar]
- 17.Mizsei J., Sipila P., Lantto V. Structural studies of sputtered noble metal catalysts on oxide surfaces. Sens. Actuators B Chem. 1998;47:139–144. doi: 10.1016/S0925-4005(98)00015-X. [DOI] [Google Scholar]
- 18.Vaishampayan M.V., Deshmukh R.G., Walke P., Mulla I.S. Fe-doped SnO2 nanomaterial: A low temperature hydrogen sulfide gas sensor. Mat. Chem. Phys. 2008;109:230–234. doi: 10.1016/j.matchemphys.2007.11.024. [DOI] [Google Scholar]
- 19.Chen Y.J., Gao X.M., Di X.-P., Ouyang Q.Y., Gao P., Qi L.H., Li C.Y., Zhu C.L. Porous Iron Molybdate Nanorods: In situ Diffusion Synthesis and Low-Temperature H2S Gas Sensing. ACS Appl. Mater. Interfaces. 2013;5:3267–3274. doi: 10.1021/am400324g. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y.L., Wang H., Yang Y., Liu Z.M., Yang H.F., Shen G.L., Yu R.Q. Hydrogen sulfide sensing properties of NiFe2O4 nanopowder doped with noble metals. Sens. Actuators B Chem. 2004;102:148–154. doi: 10.1016/j.snb.2004.04.014. [DOI] [Google Scholar]
- 21.Kapse V.D., Ghosh S.A., Raghuwanshi F.C., Kapse S.D. Nanocrystalline spinel Ni0.6Zn0.4Fe2O4: A novel material for H2S sensing. Mat. Chem. Phys. 2009;113:638–644. doi: 10.1016/j.matchemphys.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez I., Arbiol J., Dezanneau G., Cornet A., Morante J.R. Crystalline structure, defects and gas sensor response to NO2 and H2S of tungsten trioxide nanopowders. Sens. Actuators B Chem. 2003;93:475–485. doi: 10.1016/S0925-4005(03)00198-9. [DOI] [Google Scholar]
- 23.Stankova M., Vilanova X., Llobet E., Calderer J., Vinaixa M., Gracia I., Cane C., Correig X. On-line monitoring of CO2 quality using doped WO3 thin film sensor. Thin Solid Films. 2006;500:302–308. doi: 10.1016/j.tsf.2005.11.021. [DOI] [Google Scholar]
- 24.Mickelson W., Sussman A., Zettl A. Low-power, fast, selective nanoparticle-based hydrogen sulfide gas sensor. Appl. Phys. Lett. 2012;100:173110. doi: 10.1063/1.3703761. [DOI] [Google Scholar]
- 25.Kruefu V., Wisitsoraat A., Tuantranont A., Phanichphant S. Ultra-sensitive H2S sensors based on hydrothermal/impregnation-made Ru-functionalized WO3 nanorods. Sens. Actuators B Chem. 2015;215:630–636. doi: 10.1016/j.snb.2015.03.037. [DOI] [Google Scholar]
- 26.Wang C., Chu X., Wu M. Detection of H2S down to ppb levels at room temperature using sensors based on ZnO nanorods. Sens. Actuators B Chem. 2006;113:320–323. doi: 10.1016/j.snb.2005.03.011. [DOI] [Google Scholar]
- 27.Zhang N., Yu K., Li Q., Zhu Z.Q., Wan Q. Room-temperature high-sensitivity H2S gas sensor based on dendritic ZnO nanostructures with macroscale in appearance. J. Appl. Phys. 2008;103:104305. doi: 10.1063/1.2924430. [DOI] [Google Scholar]
- 28.Badadhe S.S., Mulla I.S. H2S gas sensitive indium-doped ZnO thin films: Preparation and characterization. Sens. Actuators B Chem. 2009;143:164–170. doi: 10.1016/j.snb.2009.08.056. [DOI] [Google Scholar]
- 29.Xua J., Wang X., Shen J. Hydrothermal synthesis of In2O3 for detecting H2S in air. Sens. Actuators B Chem. 2006;115:642–646. doi: 10.1016/j.snb.2005.10.038. [DOI] [Google Scholar]
- 30.Li Z., Niu X., Lin Z., Wang N., Shen H., Liu W., Sun K., Fu Y.Q., Wang Z. Hydrothermally synthesized CeO2 nanowires for H2S sensing at room temperature. J. Alloy. Compd. 2016;682:647–653. doi: 10.1016/j.jallcom.2016.04.311. [DOI] [Google Scholar]
- 31.Balamurugan C., Lee D.W. Perovskite hexagonal YMnO3 nanopowder as p-type semiconductor gas sensor for H2S detection. Sens. Actuators B Chem. 2015;221:857–866. doi: 10.1016/j.snb.2015.07.018. [DOI] [Google Scholar]
- 32.Llobet E., Molas G., Molinàs P., Calderer J., Vilanova X., Brezmes J., Sueiras J.E., Correig X. Fabrication of highly selective tungsten oxide ammonia sensors. J. Electrochem. Soc. 2000;147:776–779. doi: 10.1149/1.1393270. [DOI] [Google Scholar]
- 33.Li Y., Luo W., Qin N., Dong J., Wei J., Li W., Feng S., Chen J., Xu J., Elzatahry A.A., Es-Saheb M.-H., Deng Y., Zhao D. Highly Ordered Mesoporous Tungsten Oxides with a Large Pore Size and Crystalline Framework for H2S Sensing. Angew. Chem. Int. Ed. 2014;53:9035–9040. doi: 10.1002/anie.201403817. [DOI] [PubMed] [Google Scholar]
- 34.Roso S., Bittencourt C., Umek P., González O., Güell F., Urakawa A., Llobet E. Synthesis of single crystalline In2O3 octahedra for the selective detection of NO2 and H2 at trace levels. J. Mat. Chem. C. 2016;4:9418–9427. doi: 10.1039/C6TC03218D. [DOI] [Google Scholar]
- 35.Manorama S., Devi G.S., Rao V.J. Hydrogen sulfide sensor based on tin oxide deposited by spray pyrolysis and microwave plasma chemical vapor deposition. Appl. Phys. Lett. 1994;64:3163–3165. doi: 10.1063/1.111326. [DOI] [Google Scholar]
- 36.Tamaki J., Shimanoe K., Yamada Y., Yamamoto Y., Miura N., Yamazoe N. Dilute hydrogen sulfide sensing properties of CuO–SnO2 thin film prepared by low-pressure evaporation method. Sens. Actuators B Chem. 1998;49:121–125. doi: 10.1016/S0925-4005(98)00144-0. [DOI] [Google Scholar]
- 37.Vasiliev R.B., Rumyantseva M.N., Yakovlev N.V., Gaskov A.M. CuO:SnO2 thin film heterostructures as chemical sensors to H2S. Sens. Actuators B Chem. 1998;50:186–193. doi: 10.1016/S0925-4005(98)00235-4. [DOI] [Google Scholar]
- 38.Khanna A., Kumar R., Bhatti S.S. CuO-doped SnO2 thin films as hydrogen sulfide gas sensor. Appl. Phys. Lett. 2003;82:4388–4390. doi: 10.1063/1.1584071. [DOI] [Google Scholar]
- 39.Chowdhuri A., Gupta V., Sreenivas K., Kumar R., Mozumdar S., Patanjali P.K. Response speed of SnO2-based H2S gas sensors with CuO nanoparticles. Appl. Phys. Lett. 2004;84:1180–1182. doi: 10.1063/1.1646760. [DOI] [Google Scholar]
- 40.Kong X., Li Y. High sensitivity of CuO modified SnO2 nanoribbons to H2S at room temperature. Sens. Actuators B Chem. 2005;105:449–453. doi: 10.1016/j.snb.2004.07.001. [DOI] [Google Scholar]
- 41.Choudhary M., Singh N.K., Mishra V.N., Dwivedi R. Selective detection of hydrogen sulfide using copper oxide-doped tin oxide based thick film sensor array. Mat. Chem. Phys. 2013;142:370–380. doi: 10.1016/j.matchemphys.2013.07.030. [DOI] [Google Scholar]
- 42.Gao C., Lin Z.D., Li N., Fu P., Wang X.H. Preparation and H2S Gas-Sensing Performances of Coral-Like SnO2–CuO Nanocomposite. Acta Metall. Sin. 2015;28:1190–1197. doi: 10.1007/s40195-015-0312-y. [DOI] [Google Scholar]
- 43.Cui G., Zhang M., Zou G. Resonant tunneling modulation in quasi-2D Cu2O/SnO2 p-n horizontal multi-layer heterostructure for room temperature H2S sensor application. Sci. Rep. 2013;3:1250. doi: 10.1038/srep01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Z., Duan G., Li Y., Liu G., Zhang H., Dai Z., Cai W. CuO–ZnO Micro/Nanoporous Array-Film-Based Chemosensors: New Sensing Properties to H2S. Chem. Eur. J. 2014;20:6040–6046. doi: 10.1002/chem.201304722. [DOI] [PubMed] [Google Scholar]
- 45.Vuong N.M., Chinh N.D., Huy B.T., Lee Y.I. CuO-Decorated ZnO Hierarchical Nanostructures as Efficient and Established Sensing Materials for H2S Gas Sensors. Sci. Rep. 2016;6:26736. doi: 10.1038/srep26736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagh M.S., Patil L.A., Seth T., Amalnerkar D.P. Surface cupricated SnO2–ZnO thick films as a H2S gas sensor. Mat. Chem. Phys. 2004;84:228–233. doi: 10.1016/S0254-0584(03)00232-3. [DOI] [Google Scholar]
- 47.Annanouch F.E., Haddi Z., Vallejos S., Umek P., Guttmann P., Bittencourt C., Llobet E. Aerosol-Assisted CVD-Grown WO3 Nanoneedles Decorated with Copper Oxide Nanoparticles for the Selective and Humidity-Resilient Detection of H2S. ACS Appl. Mater. Interfaces. 2015;7:6842–6851. doi: 10.1021/acsami.5b00411. [DOI] [PubMed] [Google Scholar]
- 48.Zhang F., Zhu A., Luo Y., Tian Y., Yang J., Qin Y. CuO Nanosheets for Sensitive and Selective Determination of H2S with High Recovery Ability. J. Phys. Chem. C. 2010;114:19214–19219. doi: 10.1021/jp106098z. [DOI] [Google Scholar]
- 49.Hennemann J., Kohl C.D., Smarsly B.M., Metelmann H., Rohnke M., Janek J., Reppin D., Meyer B.K., Russ S., Wagner T. Copper oxide based H2S dosimeters—Modeling of percolation and diffusion processes. Sens. Actuators B Chem. 2015;217:41–50. doi: 10.1016/j.snb.2015.02.001. [DOI] [Google Scholar]
- 50.Kneer J., Knobelspies S., Bierer B., Wöllenstein J., Palzer S. New method to selectively determine hydrogen sulfide concentrations using CuO layers. Sens. Actuators B Chem. 2016;222:625–631. doi: 10.1016/j.snb.2015.08.071. [DOI] [Google Scholar]
- 51.Gaspera E.D., Guglielmi M., Agnoli S., Granozzi G., Post M.L., Bello V., Mattei G., Martucci A. Au Nanoparticles in Nanocrystalline TiO2-NiO Films for SPR-Based, Selective H2S Gas Sensing. Chem. Mater. 2010;22:3407–3417. doi: 10.1021/cm100297q. [DOI] [Google Scholar]
- 52.Tabassum R., Mishra S.K., Gupta B.D. Surface plasmon resonance-based fiber optic hydrogen sulphide gas sensor utilizing Cu–ZnO thin films. Phys. Chem. Chem. Phys. 2013;15:11868–11874. doi: 10.1039/c3cp51525g. [DOI] [PubMed] [Google Scholar]
- 53.Mishra S.K., Rani S., Gupta B.D. Surface plasmon resonance based fiber optic hydrogen sulphide gas sensor utilizing nickel oxide doped ITO thin film. Sens. Actuators B Chem. 2014;195:215–222. doi: 10.1016/j.snb.2014.01.045. [DOI] [Google Scholar]
- 54.Usha S.P., Mishra S.K., Gupta B.D. Fiber optic hydrogen sulfide gas sensors utilizing ZnO thin film/ZnOnanoparticles: A comparison of surface plasmon resonance and lossy mode resonance. Sens. Actuators B Chem. 2015;218:196–204. doi: 10.1016/j.snb.2015.04.108. [DOI] [Google Scholar]
- 55.Usha S.P., Mishra S.K., Gupta B.D. Zinc oxide thin film/nanorods based lossy mode resonance hydrogen sulphide gas sensor. Mater. Res. Express. 2015;2:095003. doi: 10.1088/2053-1591/2/9/095003. [DOI] [Google Scholar]
- 56.Fam D.W.H., Tok A.I.Y., Palaniappan A., Nopphawan A.P., Lohani A., Mhaisalkar S.G. Selective sensing of hydrogen sulphide using silver nanoparticle decorated carbon nanotubes. Sens. Actuators B Chem. 2009;138:189–192. doi: 10.1016/j.snb.2009.01.008. [DOI] [Google Scholar]
- 57.Mubeen S., Zhang T., Chartuprayoon N., Rheem Y., Mulchandani A., Myung N.V., Deshusses M.A. Sensitive Detection of H2S Using Gold Nanoparticle Decorated Single-Walled Carbon Nanotubes. Anal. Chem. 2010;82:250–257. doi: 10.1021/ac901871d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zanolli Z., Leghrib R., Felten A., Pireaux J.J., Llobet E., Charlier J.C. Gas Sensing with Au-Decorated Carbon Nanotubes. ACS Nano. 2011;5:4592–4599. doi: 10.1021/nn200294h. [DOI] [PubMed] [Google Scholar]
- 59.Moon S., Vuong N.M., Lee D., Kim D., Lee H., Kim D., Hong S.K., Yoon S.G. Co3O4–SWCNT composites for H2S gas sensor application. Sens. Actuators B Chem. 2016;222:166–172. doi: 10.1016/j.snb.2015.08.072. [DOI] [Google Scholar]
- 60.Asad M., Sheikhi M.H. Surface acoustic wave based H2S gas sensors incorporating sensitive layers of single wall carbon nanotubes decorated with Cu nanoparticles. Sens. Actuators B Chem. 2014;198:134–141. doi: 10.1016/j.snb.2014.03.024. [DOI] [Google Scholar]
- 61.Bhadra N., Hussain S., Das S., Bhunia R., Bhar R., Pal A.K. H2S Gas Sensor Based on Nanocrystalline Copper/DLC Composite Films. Plasmonics. 2015;10:503–509. doi: 10.1007/s11468-014-9834-9. [DOI] [Google Scholar]
- 62.Zhou L., Shen F., Tian X., Wang D., Zhang T., Chen W. Stable Cu2O nanocrystals grown on functionalized graphene sheets and room temperature H2S gas sensing with ultrahigh sensitivity. Nanoscale. 2013;5:1564–1569. doi: 10.1039/c2nr33164k. [DOI] [PubMed] [Google Scholar]
- 63.MalekAlaie M., Jahangiri M., Rashidi A.M., HaghighiAsl A., Izadi N. Selective hydrogen sulfide (H2S) sensors based on molybdenum trioxide (MoO3) nanoparticle decorated reduced graphene oxide. Mat. Sci. Semicond. Proc. 2015;38:93–100. doi: 10.1016/j.mssp.2015.03.034. [DOI] [Google Scholar]
- 64.Cho S., Lee J.S., Jun J., Kim S.G., Jang J. Fabrication of water-dispersible and highly conductive PSS-doped PANI/graphene nanocomposites using a high-molecular weight PSS dopant and their application in H2S detection. Nanoscale. 2014;6:15181–15195. doi: 10.1039/C4NR04413D. [DOI] [PubMed] [Google Scholar]
- 65.Alaie M.M., Jahangiri M., Rashidi A.M., Asl A.H., Izadi N. A novel selective H2S sensor using dodecylamine and ethylenediamine functionalized graphene oxide. J. Ind. Eng. Chem. 2015;29:97–103. doi: 10.1016/j.jiec.2015.03.021. [DOI] [Google Scholar]
- 66.Rani S., Kumar M., Garg R., Sharma S., Kumar D. Amide Functionalized Graphene Oxide Thin Films for Hydrogen Sulfide Gas Sensing Applications. IEEE Sens. J. 2016;16:2929–2934. doi: 10.1109/JSEN.2016.2524204. [DOI] [Google Scholar]
- 67.Li X., Jiang Y., Xie G., Taia H., Sunb P., Zhang B. Copper phthalocyanine thin film transistors for hydrogen sulfide detection. Sens. Actuators B Chem. 2013;176:1191–1196. doi: 10.1016/j.snb.2012.09.084. [DOI] [Google Scholar]
- 68.Collins R.A., Mohammed K.A. Gas sensitivity of some metal phthalocyanines. J. Phys. D: Appl. Phys. 1998;21 doi: 10.1088/0022-3727/21/1/021. [DOI] [Google Scholar]
- 69.Kumar A., Brunet J., Varenne C., Ndiaye A., Pauly A. Phthalocyanines based QCM sensors for aromatic hydrocarbons monitoring: Role of metal atoms and substituents on response to toluene. Sens. Actuators B Chem. 2016;230:320–329. doi: 10.1016/j.snb.2016.02.032. [DOI] [Google Scholar]
- 70.Bai S., Zhang K., Sun J., Zhang D., Luo R., Li D., Liu C. Polythiophene-WO3 hybrid architectures for low-temperature, H2S detection. Sens. Actuators B Chem. 2014;197:142–148. doi: 10.1016/j.snb.2014.02.038. [DOI] [Google Scholar]
- 71.Liu H., Li M., Shao G., Zhang H., Wang W., Song H., Cao H., Ma W., Tang J. Enhancement of hydrogen sulfide gas sensing of PbS colloidal quantum dots by remote doping through ligand exchange. Sens. Actuators B Chem. 2015;212:434–439. doi: 10.1016/j.snb.2015.02.047. [DOI] [Google Scholar]
- 72.Li M., Zhou D., Zhao J., Zheng Z., He J., Hub L., Xia Z., Tang J., Liu H. Resistive gas sensors based on colloidal quantum dot (CQD) solids for hydrogen sulfide detection. Sens. Actuators B Chem. 2015;217:198–201. doi: 10.1016/j.snb.2014.07.058. [DOI] [Google Scholar]
- 73.Wan X., Wu L., Zhang L., Song H., Lv Y. Novel metal-organic frameworks-based hydrogen sulfide cataluminescence sensors. Sens. Actuators B Chem. 2015;220:614–621. doi: 10.1016/j.snb.2015.05.125. [DOI] [Google Scholar]
- 74.Drobek M., Kim J.H., Bechelany M., Vallicari C., Julbe A., Kim S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces. 2016;8:8323–8328. doi: 10.1021/acsami.5b12062. [DOI] [PubMed] [Google Scholar]
- 75.Kumar N., Bhalla V., Kumar M. Recent developments of fluorescent probes for the detection of gasotransmitters (NO, CO and H2S) Coord. Chem. Rev. 2013;257:2335–2347. doi: 10.1016/j.ccr.2013.02.028. [DOI] [Google Scholar]
- 76.Chen S., Chen Z., Ren W., Ai H. Reaction-Based Genetically Encoded Fluorescent Hydrogen Sulfide Sensors. J. Am. Chem. Soc. 2012;134:9589–9592. doi: 10.1021/ja303261d. [DOI] [PubMed] [Google Scholar]
- 77.Nagarkar S.S., Desai A.V., Ghosh S.K. A Nitro-Functionalized Metal–Organic Framework as a Reaction-Based Fluorescence Turn-On Probe for Rapid and Selective H2S Detection. Chem. Eur. J. 2015;21:9994–9997. doi: 10.1002/chem.201501043. [DOI] [PubMed] [Google Scholar]
- 78.Coles G.S.V., Williams G., Smith B. The effect of oxygen partial pressure on the response of tin (IV) oxide based gas sensors. J. Phys. D Appl. Phys. 1991;24:633–641. doi: 10.1088/0022-3727/24/4/017. [DOI] [Google Scholar]
- 79.Loloee R., Chorpening B., Beer S., Ghosh R.N. Hydrogen monitoring for power plant applications using SiC sensors. Sens. Actuators B Chem. 2008;129:200–210. doi: 10.1016/j.snb.2007.07.118. [DOI] [Google Scholar]
- 80.Wang B., Wang Y., Lei Y., Xie S., Wu N., Gou Y., Han C., Shi Q., Fang D. Vertical SnO2 nanosheet-SiC nanofibers with hierarchical architecture for high-performance gas sensors. J. Mat. Chem. C. 2016;4:295–304. doi: 10.1039/C5TC02792F. [DOI] [Google Scholar]
- 81.Gaiardo A., Bellutti P., Fabbri B., Gherardi S., Giberti A., Guidi V., Landini N., Malagù C., Pepponi G., Valt M., et al. Chemoresistive Gas Sensor based on SiC Thick Film: Possible Distinctive Sensing Properties between H2S and SO2. Proceedoa Eng. 2016;168:276–279. doi: 10.1016/j.proeng.2016.11.191. [DOI] [Google Scholar]