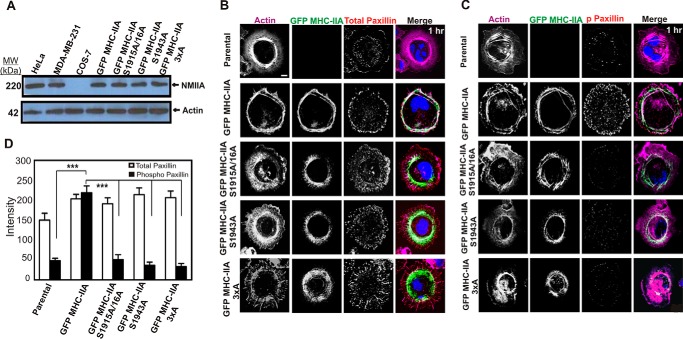
FIGURE 1.
C-terminal NMIIA phosphorylation sites are critical for recruitment of NMIIA to lamellar protrusions and for marginal paxillin phosphorylation during active cell spreading. A, whole-cell lysates of HeLa, MDA-MB-231, COS-7, and COS-7 cells expressing the indicated GFP MHC-IIA construct were subjected to Western blotting analysis with anti-MHC-IIA and anti-actin antibodies. B and C, parental COS-7 cells and COS-7 cells expressing the indicated GFP MHC-IIA construct were allowed to spread for 60 min on collagen I-coated glass, fixed, and stained with Alexa-568-phalloidin to visualize F-actin (violet) and DAPI to visualize nuclei (blue) and immunostained with anti-paxillin antibody (B) or anti-phospho-Tyr-118 paxillin antibody (C) (red). Scale bar, 20 μm. D, quantitation of paxillin versus phospho-paxillin staining in actively spreading cells. All images were obtained by confocal laser scanning microscopy and are from confocal slices taken within 2 μm of the substratum (n = 6 cells, data pooled from two different experiments performed on different dates). Data were plotted as mean ± S.D. *** indicated phospho-paxillin in GFP-MHC IIA differs from all other lines, p < 0.001.