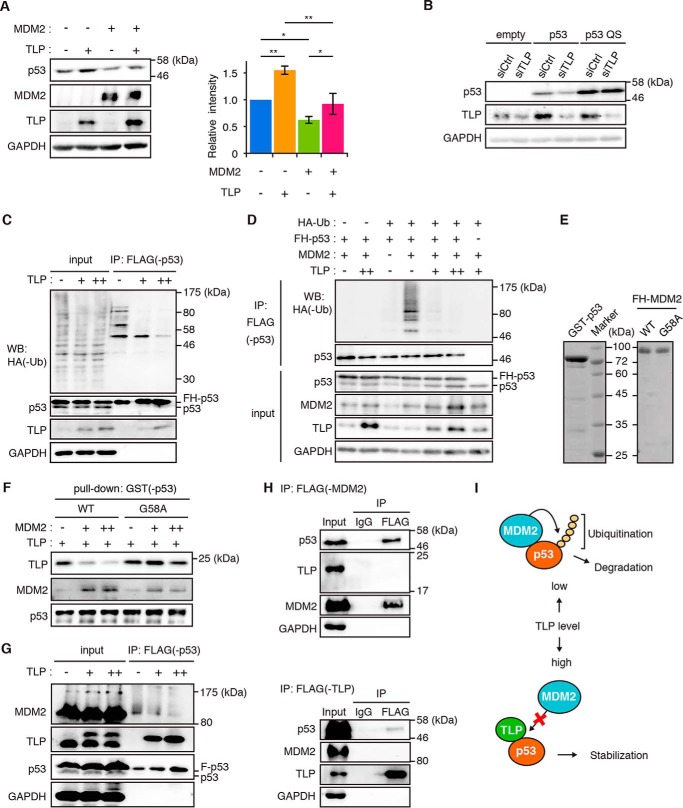
FIGURE 4.
TLP suppresses p53 ubiquitination and interferes with the p53-MDM2 interaction. A, HCT116 cells were transfected with MDM2 and TLP expression vectors. Left, 35 h after transfection, the amounts of the indicated proteins were determined. Right, relative band intensities of p53 are displayed (mean ± S.D., n = 4). *, p < 0.05; **, p < 0.01 (Tukey's honestly significant difference test). B, HCT116 p53-null cells were transfected with wild-type p53 (p53) or MDM2 binding ability-defective mutant p53 (p53 QS) together with siTLP. p53 protein was detected with polyclonal p53 antibody. C, HCT116 cells were transfected with expression vectors for FH-p53, HA-ubiquitin (Ub), and increased amounts of TLP. Twenty-four hours after transfection the cells were treated with MG132 for 3 h. The whole cell lysate was immunoprecipitated (IP) with anti-FLAG-Sepharose beads. p53-bound HA-tagged ubiquitin (HA-Ub) was detected by Western blot (WB) using anti-HA antibody. D, HCT116 cells were transfected with expression vectors for FH-p53, HA-Ub, MDM2, and increased amounts of TLP. Twenty-four hours after transfection the cells were treated with MG132 for 3 h. Then FH-p53 in whole cell lysate was immunoprecipitated with anti-FLAG-Sepharose beads, and p53-bound ubiquitin was detected. E, Escherichia coli-expressed GST-p53, FH-MDM2 (WT), and FH-MDM2 mutant (G58A) were purified from cell extracts using tag affinity resins. Proteins were stained with Coomassie Brilliant Blue. F, increased amounts of FH-MDM2 or FH-G58A were added to constant amounts of glutathione-Sepharose-bound GST-p53 and FH-TLP. Pull-downed proteins were detected by Western blot with corresponding antibodies. G, expression vectors for FH-p53 and increased amount of HA-TLP were introduced into HCT116 cells. FH-p53 in cell lysates was immunoprecipitated with anti-FLAG-Sepharose beads and eluted with a FLAG-peptide. p53, p53-bound MDM2, and p53-bound TLP were then detected. H, HCT116 cells were transfected with expression vectors for FH-MDM2 or FH-TLP together with HA-p53. Twenty-four hours after transfection, the cells were treated with MG132 for 2 h. FH-MDM2 (upper) and TLP (lower) in whole cell lysate was immunoprecipitated with anti-FLAG-Sepharose beads. I, schematic model of the interplay between p53, MDM2, and TLP. Low levels of TLP resulted in a situation whereby MDM2 promotes p53 ubiquitination and degradation. When TLP levels are elevated (e.g. in response to genotoxic stress), TLP binds to and stabilizes p53 by releasing p53 from MDM2-mediated negative control.