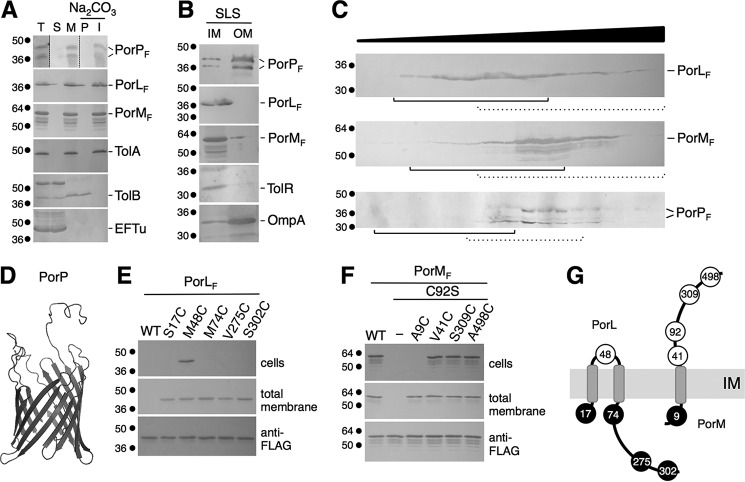
FIGURE 2.
Localization and topologies of the PorL, PorM, PorN, and PorP proteins. A, PorL, PorM, and PorP co-fractionate with integral membrane proteins. E. coli cells producing FLAG-tagged PorL, PorM, or PorP (T, total fraction) were fractionated to separate soluble (S) and membrane (M) fractions. Membranes were then treated with sodium carbonate (Na2CO3) to separate peripheral (P) and integral (I) membrane proteins. Samples from 5 × 108 cells were subjected to 12.5% SDS-PAGE and immunodetected with antibodies directed against the EFTu (soluble), TolB (soluble and peripherally associated with the membrane), and TolA (integral inner membrane) proteins and the FLAG epitope. B, total membranes from E. coli cells producing FLAG-tagged PorP, PorL, or PorM were subjected to solubilization with SLS. Solubilized IM and insolubilized OM proteins were separated. Samples from 5 × 108 cells were subjected to 12.5% SDS-PAGE and immunodetected with antibodies directed against the TolR (inner membrane) and OmpA (outer membrane) proteins and the FLAG epitope. C, total membranes from E. coli cells producing FLAG-tagged PorL, PorM, or PorP were separated on a discontinuous sedimentation sucrose gradient. The collected fractions were analyzed for content using anti-FLAG antibodies. The positions of the inner (plain lines) and outer membrane (dotted lines) fractions, based on immunodetection controls with anti-TolA (inner membrane) and anti-OmpF/anti-Pal (outer membrane) antibodies and with an NADH oxidase (inner membrane) activity test (supplemental Fig. S4), are indicated. Molecular weight markers are indicated on the left. D, homology model of the PorP protein based on the crystal structure of the R. pickettii toluene transporter TbuX protein (PDB code 3BRY), generated using HHPred/Swiss-Model. E and F, accessibility of cysteine residues. Whole cells (top panels) or total membranes (center panel) of E. coli cells producing the FLAG-tagged PorL (E) or PorM (F) WT or cysteine-substituted derivatives were treated with the MPB probe and solubilized, and the PorL and PorM proteins were immunoprecipitated using agarose beads coupled to M2 anti-FLAG antibody. Precipitated material was subjected to SDS-PAGE and Western blotting analysis using anti-FLAG antibody (to detect PorL or PorM, bottom panels) and streptavidin coupled to alkaline phosphatase (to detect biotinylated PorL or PorM derivatives). Molecular weight markers are indicated on the left. G, topology model for the PorL and PorM proteins at the inner membrane based on the cysteine accessibility experiments. The positions of the labeled and unlabeled cysteine residues are indicated by open and filled circles, respectively.