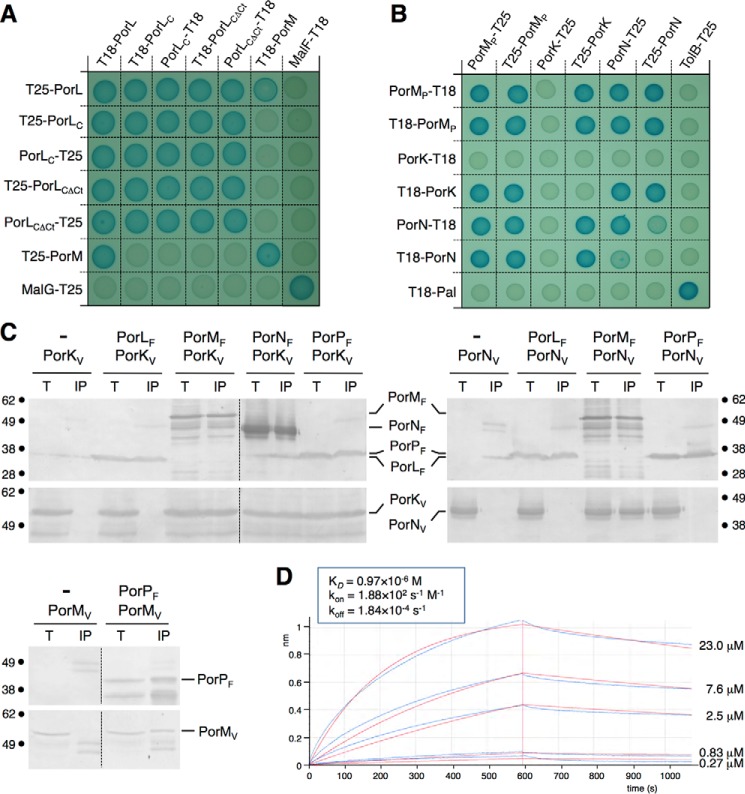
FIGURE 3.
Interaction network among the PorKLMNP complex. A and B, bacterial two-hybrid assay. BTH101 reporter cells producing the indicated proteins or domains (PorLC, cytoplasmic domain of the PorL protein; PorLCΔCt, cytoplasmic domain of the PorL protein deleted from the C-terminal hydrophobic helix) fused to the T18 or T25 domain of the Bordetella adenylate cyclase were spotted on plates supplemented with IPTG and the chromogenic substrate X-gal. Interaction between the two fusion proteins is attested by the dark blue color of the colony. The MalF-MalG (A) and TolB-Pal (B) interactions served as positive controls. C, co-immunoprecipitation assays. Detergent-solubilized extracts of E. coli cells producing the indicated VSVG- and FLAG-tagged proteins were subjected to immunoprecipitation with anti-FLAG-coupled beads. The input (total soluble material, T) and the immunoprecipitated material (IP) were loaded on a 12.5% SDS-PAGE and immunodetected with anti-FLAG and anti-VSVG monoclonal antibodies. The immunodetected proteins and the molecular weight markers are indicated. D, biolayer interferometry. PorM and PorN interact with a KD of 0.97 μm. The recordings represent binding of the indicated concentration of purified PorMP to the sensor tip coupled to purified PorN (experimental and fitted curves are shown in blue and red, respectively). The response (in nanometers) is plotted versus time (in seconds). The kinetics parameters are indicated in the inset.