FIGURE 7.
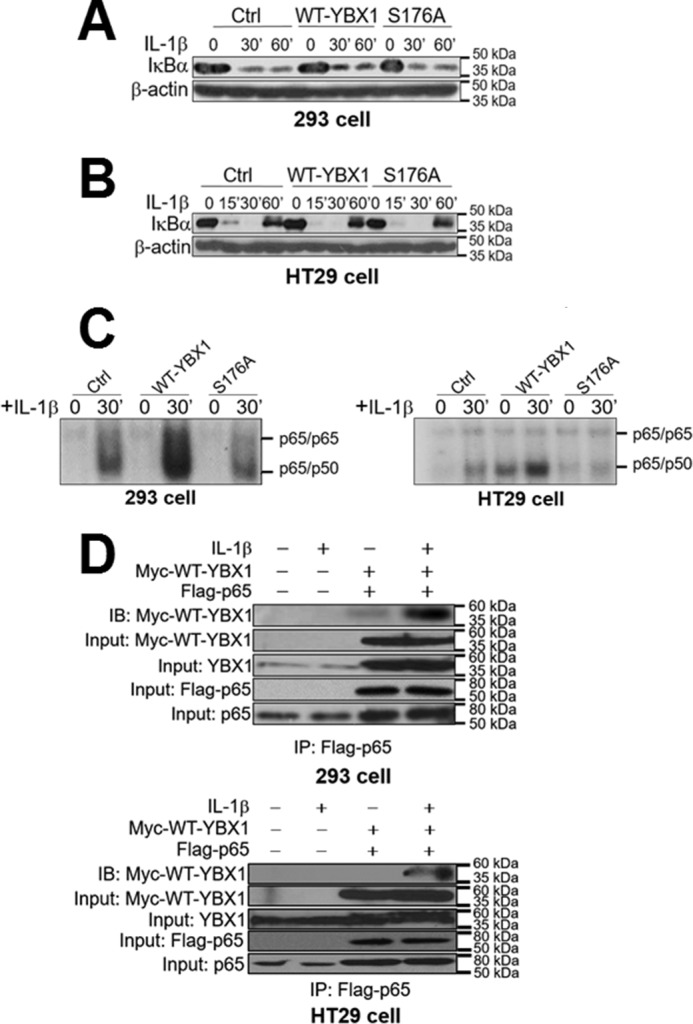
Activation of NF-κB by YBX1 Ser-176 phosphorylation works independently of IκBα degradation. A and B, Western blots in 293 (A) and HT29 cells (B) showing that there is no significant difference for the degradation pattern of IκBα after IL-1β treatment among Ctrl, WT-YBX1, and S176A mutant cells. C, EMSA assay showing that overexpression of WT-YBX1 enhanced NF-κB (mainly p65/p50 heterodimer) DNA binding ability compared with Ctrl in both 293 (left panel) and HT29 cells (right panel), whereas the S176A mutant showed decreased NF-κB DNA binding ability compared with WT-YBX1. IL-1β-induced (30-min treatment) NF-κB binding in Ctrl cells served as the positive control, which has been published previously (1, 12, 13, 36, 37). D, top panel, co-immunoprecipitation (IP) experiments in 293 cells with or without co-expression of Myc-tagged WT-YBX1 and FLAG-tagged p65. These cells were treated with IL-1β for 1 h or left untreated. FLAG-p65 was then pulled down with anti-FLAG beads. Samples were then subjected to Western blotting analysis (immunoblotting, IB) and probed with anti-Myc antibody to detect the co-immunoprecipitation of Myc-WT-YBX1. The data indicated that IL-1β treatment enhanced the interaction between Myc-WT-YBX1 and FLAG-p65 when both Myc-WT-YBX1 and FLAG-p65 were co-expressed. For inputs, anti-Myc antibody was used to detect Myc-WT-YBX1, anti-YBX1 antibody was used to show the input of total YBX1, anti-FLAG antibody was used to detect the input of FLAG-p65, and anti-p65 antibody was used to show the input of total p65. Bottom panel, similar experiments were done in HT29 cells and showed similar results. The conditions and denotations are same as in the top panel.
