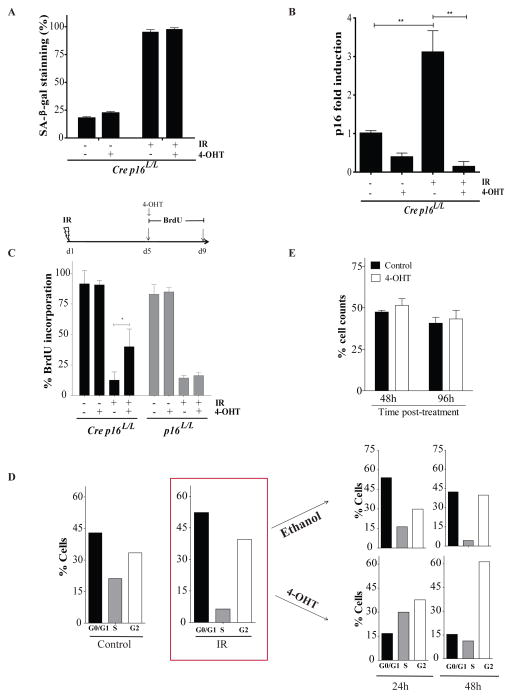
Figure 1.
Deletion of p16INK4a in irradiated bone marrow stromal cells allows for cell cycle progression but not cell growth. (a) Proportion of cells staining positive for SA-β-galactosidase (SA-β-gal) 9 days post-exposure or not to 10 Gy IR. Where indicated, cells were treated (+) or not (−) with 100nM 4-Hydroxy Tamoxifen (4-OHT) overnight on day 5 post-IR. Bone marrow stromal cells expressing or not the Cre recombinase (defined as Cre p16L/L or p16L/L respectively) were isolated has previously described4 from the femur of p16INK4a specific conditional allele transgenic mice. Cells were used at low passage (less than 3) and cultured in DMEM containing 10% fetal bovine serum under low (3%) oxygen concentration. (b) Differential mRNA expression levels of p16INK4a as determined by qPCR in Cre p16L/L cells treated as described above. Shown is fold increase in p16INK4a expression normalized to 18S. Student t-test (** p < 0.01). q-PCR was performed using SYBR GREEN PCR SensiMix™ low ROX kit (Quantance, CA, USA) using the following primers for p16INK4a and S18 genes F5′AACTCTTTCGGTCGTACCCC3′, R5′GCGTGCTTGAGCTGAAGCTA3′ and F5′TCAACTTTCGATGGTAGTCGCCGT3′, R5′TCCTTGGATGTGGTAGCCGTTTCT3′ respectively. (c) Proportion of cells incorporating BrdU (4-day pulse) 5 days after exposure or not to IR as determined by immunostaining (BrdU antibody catalogue number 347583, BD Biosciences, USA). Inactivation of p16INK4a by 4-OHT was initiated simultaneously with addition of BrdU. Student t-test (* p < 0.05). (d) Cell cycle analysis of Cre p16L/L cells before and 5 days post exposure to IR as determined by flow cytometry. Irradiated cells were then treated with 4-OHT or ethanol (vehicle) and cell cycle analysed again 24 and 48 hours later. Shown are results of a representative experiment from n=3 independent cell populations. (e) Cre p16L/L cells were irradiated and 5 day later the cells were treated with 4-OHT or its vehicle. The proportion of viable cells was determined 48 and 96 hours later. Data are expressed as mean ± SD of n=3 independent cell populations.