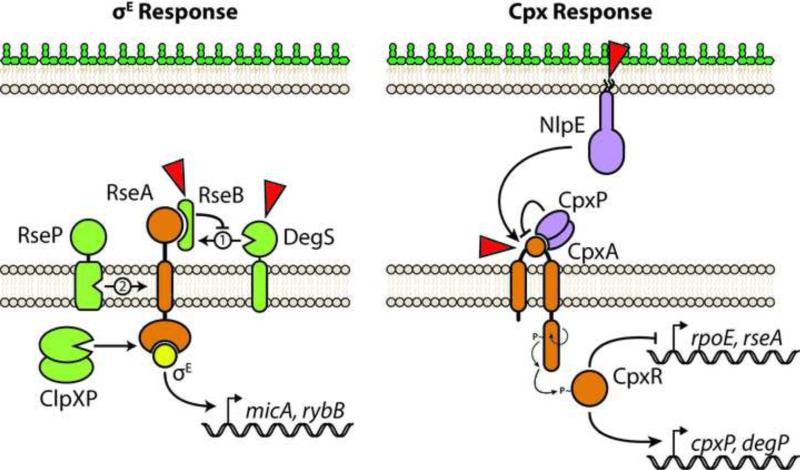
Figure 2. The σE and Cpx envelope stress responses of E. coli.
Left, the σE response is activated by a series of proteolysis steps that degrade the anti-σ factor RseA and liberate σE into the cytosol where it can increase target gene transcription. The RseA periplasmic domain is first cleaved by DegS. RseB binds RseA and inhibits DegS activity. DegS senses unfolded OMP cues. RseB is proposed to be displaced from RseA in response to LPS binding. RseB displacement allows DegS to cleave RseA and initiate the response. RseA is subsequently cleaved by the intramembrane protease RseP. A cytoplasmic portion of RseA is then degraded by the ClpXP protease to release σE. Right, the Cpx TCS responds to numerous signals detected by the periplasmic sensory domain of CpxA. CpxP inhibits CpxA activation likely by interacting with the sensory domain directly. NlpE can transduce cell adhesion signals across the envelope to activate the Cpx response. Stimulated CpxA histidine kinase autophosphorylates and transfers the phosphate to the CpxR response regulator, which alters gene expression. Sensory inputs are illustrated by red triangles.