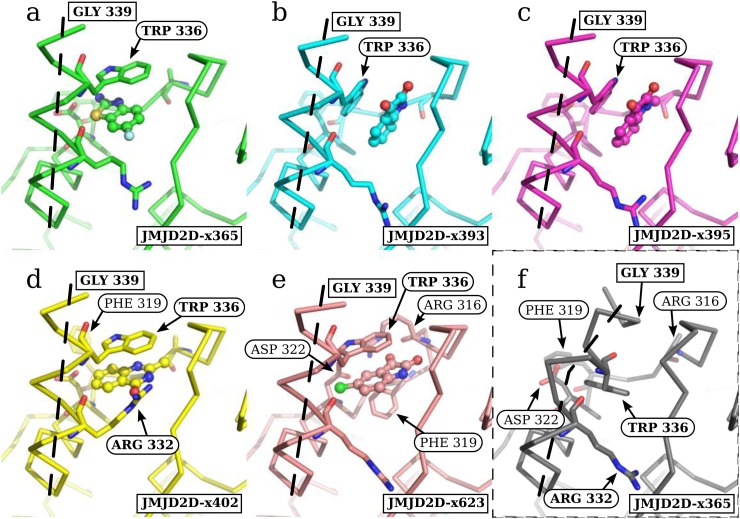
FIG. 8.
The ligands consistently move the C-terminal helix, but two distinct structural sub-states are observed. (a)–(e) Open fragment-stabilized conformations of the C-terminal helix. (f) Unbound conformation. TRP 336 adopts two distinct sub-states, where the rotamer is (a), (d), (e) changed and (b), (c) unchanged from the (f) unbound conformation. The path of the terminal helix is marked with a dashed line. Interesting residues are labelled in each structure, though the same set of residues are shown as sticks in each image: ARG316, PHE319, ASP322, ARG332, and TRP336. Residues ARG 316, PHE 319, and ASP 322 are more thoroughly discussed in supplementary material, Figure A2. The end of the modelled helix for the bound states (GLY339) is shown in all images. Images: PyMOL.