Figure 2.
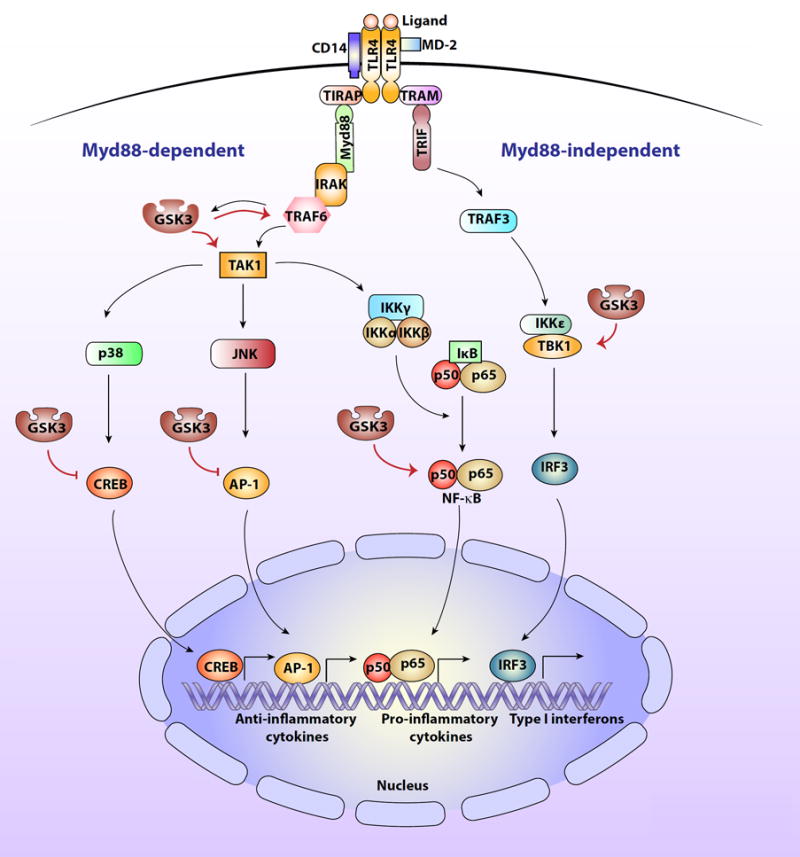
The involvement of GSK3 in TLR4 signaling pathways.
Activation of TLR4 by LPS or alarmins (called ligand in the figure) involves dimers of TLR4 binding to CD14 and MD-2. This induces Myd88 (myeloid differentiation factor 88)-dependent signaling to two serine-threonine kinases, IRAK4 (IL-1-receptor associated kinase 4) and IRAK1, which in turn bind to the E3 ubiquitin ligase TRAF6 (TNF receptor-associated factor 6) leading to activation of the serine-threonine kinase TAK1 (transforming growth factor beta-activated kinase 1), which is a site of regulation by GSK3. TAK1 phosphorylates the IκB kinase complex (IKK), which activates IKK. IKK in turn phosphorylates IκB, which releases active NF-κB. NF-κB, activation of which is promoted by GSK3, then migrates to the nucleus and activates the transcription of proinflammatory cytokines. TAK1 also activates the kinases p38 and JNK, which activate other transcription factors, such as AP-1 (activator protein 1), and CREB (cyclic AMP response element binding protein) that are involved in the production of anti-inflammatory cytokines and are inhibited by GSK3. Activated TLR4 also signals through a Myd88-independent pathway via TRAM (TRIF-related adaptor molecule) and TRIF (TIR domain-containing adaptor-inducing IFN-β) to activate TRAF3 (TNF receptor-associated factor 3), which in turn activates the serine/threonine kinase, TBK1 (TANK Binding kinase 1), which is regulated by GSK3. TBK1 promotes activation of the transcription factor IRF3 (interferon regulatory factor-3), which translocates to the nucleus where it induces the production of type I interferons.
