Fig. 3.
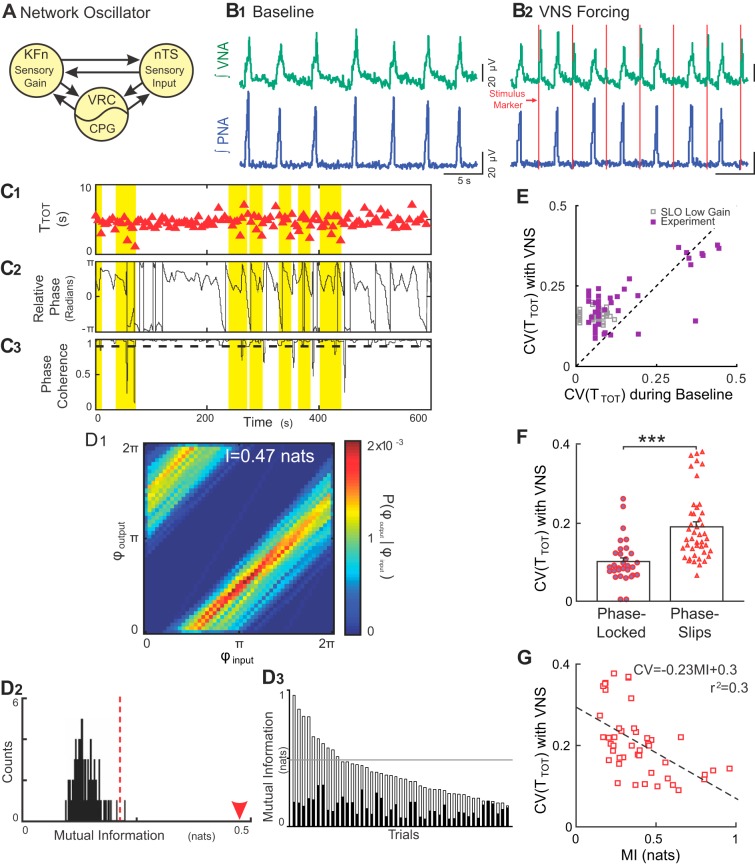
Rhythmic VNS evokes stable input-output entrainment and increases respiratory rhythm variability in situ. A: schematic model of the Hering-Breuer reflex circuit is shown. The nucleus tractus solitarii (nTS) relays vagal sensory input to the lateral respiratory column, specifically, the ventral respiratory column (VRC) including the central pattern generator (CPG) and Kölliker-Fuse nuclei (KFn). These three regions are connected reciprocally and can be thought of collectively as a single oscillator when analyzing phrenic motor output. B: representative traces of PNA and VNA are shown during baseline (B1) and VNS (B2). B1: at baseline, the in situ arterially perfused preparation generated an in vivo-like pattern of activities, wherein VNA peaked after PNA. B2: during VNS (red lines), each train of VNS evoked bursts of efferent VNA. C: representative plots of the respiratory period (C1, TTOT), instantaneous relative phase (C2, ϕoutput-ϕinput) and phase coherence during VNS (C3) are shown. Flat regions in the instantaneous relative phase (C2) indicate periods of perfect phase locking. The phase coherence (C3) measures the mean direction of the relative phase time series in a 20-s sliding window. A phase slip event was defined as a window with a phase coherence less than 0.9 (dashed line in C3) and are highlighted across all three plots. Importantly, the variability in the respiratory period increased selectively during phase slip events. D: joint probability histogram (D1) of the instantaneous phase of the output (PNA; ϕoutput) given the instantaneous phase of the input (VNS stimulus; ϕinput) had strong banding, indicative of stable input-output entrainment. The mutual information for this epoch was 0.47 nats (arrowhead in D2). To determine the significance of the entrainment interaction, the data were bootstrapped by shuffling the interbreath intervals (n = 100) and recomputing the mutual information of the instantaneous phases (D2). The observed value (arrowhead) was greater than the 99% confidence interval of the bootstrapped distribution (dashed line), indicating that the observed input-output entrainment was significant. The mutual information of the instantaneous phases (open bars) and their corresponding 99% confidence intervals (solid bars) was significant for 44 of 45 trials from eight experiments (D3). The distribution of the mutual information of the instantaneous phases derived from simulations of the low-gain SLO model (gray lines) fell slightly above the median of the experimental data set. E: VNS increased the coefficient of variation (CV) of the instantaneous period (purple squares) (Wilcoxon signed-rank test; P = 2.14 × 10–5). For qualitative comparison, we have also plotted the distribution of CVs from the simulations of the low-gain SLO model (gray squares), which overlap with the experimental distribution. F: for the group of experiential preparations, the CV during phase-locked windows was less than the CV during phase-slip windows, suggesting that the source of the increase in variability during VNS was the phase slip events (***P < 0.001). G: accordingly, the strength of entrainment during rhythmic VNS (as quantified by mutual information) was inversely correlated with the magnitude of respiratory rhythm variability. Together, these results are consistent with the predictions of the low-gain, forced, stochastic SLO model, suggesting that the intact respiratory rhythm-generating network similarly maintains a low-input gain for vagal afferent input.