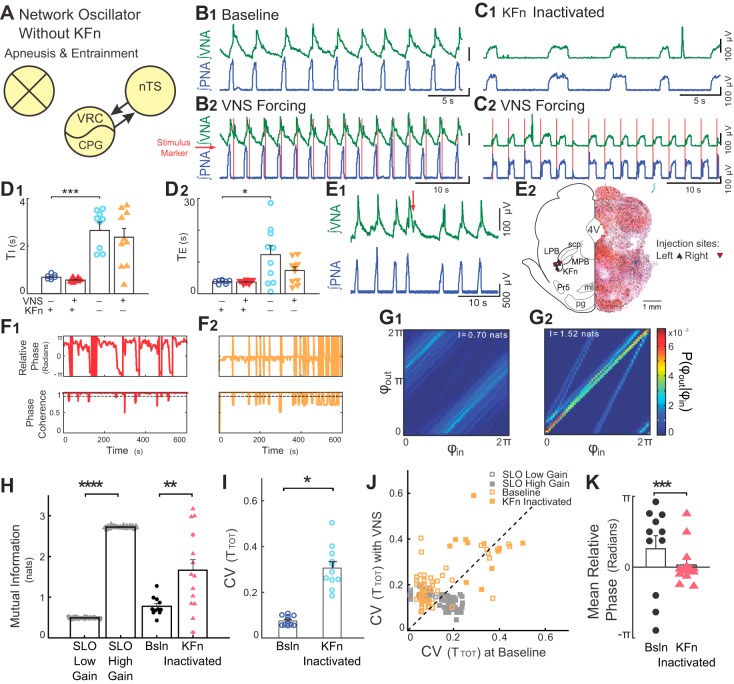
Fig. 5.
Suppression of KFn activity evokes high-gain responses to rhythmic VNS. A: suppression of KFn activity spared nTS-CPG connectivity selectively and was sufficient to switch the network state from a low- to high-gain state. B and C: representative traces of PNA and VNA are shown during baseline (B1 and C1) and VNS (B2 and C2) before (B) and after (C) microinjection of muscimol in the KFn. C: suppression of KFn activity increased TI and TE and transformed the respiratory rhythm to a two-phase rhythm without postinspiratory output on vagal efferent fibers. During rhythmic VNS, entrainment strength increased, and the phase angle between VNS and respiration cycle shifted from postinspiratory to preinspiratory coupling. D: for the group, silencing the KFn increased TI (D1, ***P < 0.001) and TE (D2, *P < 0.05). E: respiratory related dorsolateral pontine subnuclei were identified during the experiment by the respiratory responses to local glutamate microinjection (E1) and after the experiment by histological identification of the injection site (E2). E1: injections of glutamate (20–50 nl, 10 mM, at red arrow) at the target site evoked VNA and prolonged TE. E2, right: a representative hemisection counterstained with neutral red shows a KFn-targeted muscimol microinjection site marked by pontamine sky blue. E2, left: locations of the recovered microinjection sites for experiments included in the subsequent analyses are shown. Left and right injection sites are indicated by upward- and downward-pointing triangles, respectively. 4V, fourth ventricle; KFn, Kölliker-Fuse nucleus; LPB, lateral parabrachial nucleus; MPB, medial parabrachial nucleus; ml, medial lemniscus; Pr5, principal sensory of CN V; pg, pontine gray; scp, superior cerebellar peduncle. F: instantaneous relative phase (top) and phase coherence (bottom) are shown before (F1) and after (F2) microinjection of muscimol in the KFn. After suppression of KFn, respiration entrained to VNS with a relative phase near 0 (i.e., the start of inspiration). The reduction in respiratory drive evoked by suppressing KFn activity caused a qualitative change in the desynchronization events, such that they occurred abruptly when respiratory drive was not great enough to evoke an inspiratory burst. G: nonetheless, joint probability histograms of the instantaneous phases before (G1) and after (G2) microinjection of muscimol in the KFn suggest the presence of stronger entrainment after muscimol microinjection. H: for the group, KFn interventions increased the strength entrainment as measured by the mutual information of the instantaneous phases (**P < 0.01), which is consistent with a transition of the state of the network oscillator from low to high gain. For qualitative comparison, we have replotted the effect of increasing gain from the SLO model (****P < 0.001; see Fig. 2). Note that starred data points indicate VNS stimulation trials that failed the bootstrap test. I: at baseline, in the absence of VNS, suppression of KFn activity with muscimol significantly increased the variability in TTOT (*P < 0.05). J: VNS stimulation amplified respiratory rhythm variability before (open orange squares; P < 10–3), but not after (solid orange squares; P = 0.15) KFn muscimol microinjections. For qualitative comparison, we have also plotted the distribution of CVTOT) from simulations of the low- (open gray squares) and high- (solid gray squares) gain SLO models (see Fig. 2). In both model and experimental data, the perturbation (change of gain, or muscimol microinjection) shifted the distribution to lie along the line of identity, suggesting that the amplification of rhythm variability no longer occurred. However, the model failed to capture the increase in rhythm variability evoked by KFn muscimol microinjection. K: suppression of KFn activity shifted the preferred phase during entrainment from a postinspiratory to a preinspiratory relative phase angle (***P = ≪ 0.0001). Together, these observations suggest that activity in the KFn regulates the gain of vagal inputs and is responsible for the amplification of respiratory rhythm variability during VNS forcing.