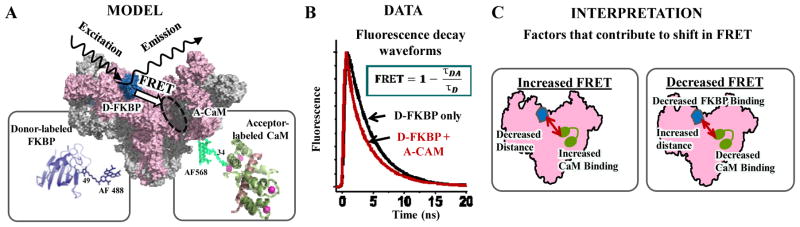
Figure 1. FRET-based HTS-compatible system to monitor RyR interaction with CaM and FKBP.
(A) Schematic illustration of the FKBP and CaM binding locations within the RyR cryo-EM map (16,17), and donor and acceptor label positions on ribbon-structures of FKBP and CaM, respectively (PDB 4IQ2 and 2BCX). (B) Time-resolved (TR) fluorescence waveforms yield the fluorescence lifetime (FLT) readout that enables high-precision measurements of FRET between D-FKBP and A-CaM. FRET is calculated as the fractional change between the fluorescence lifetimes of donor-only and donor+acceptor sample lifetime (τD and τDA, respectively). (C) FRET changes can be due to alterations in FKBP or CaM binding affinity and/or RyR structure that affects distance relationships between fluorophores.