Abstract
Many epidemiological and clinical studies use accelerometry to objectively measure physical activity using the activity counts, vector magnitude, or number of steps. These measures use just a fraction of the information in the raw accelerometry data as they are typically summarized at the minute level. To address this problem, we define and estimate two measures of temporal stride-to-stride gait variability based on raw accelerometry data: Amplitude Deviation (AD) and Phase Deviation (PD). We explore the sensitivity of our approach to on-body placement of the accelerometer by comparing hip, left and right wrist placements. We illustrate the approach by estimating AD and PD in 46 elderly participants in the Developmental Epidemiologic Cohort Study (DECOS) who worn accelerometers during a 400 meter walk test. We also show that AD and PD have a statistically significant association with the gait speed and sit-to-stand test performance.
Keywords: accelerometry, gait asymmetry, wearable computing, stride variability
1 Introduction
Accelerometers are now ubiquitous in health studies, where they are used to provide objective and reproducible proxy measurements of physical activity. Examples of such studies include both large surveillance and epidemiological cohorts, such as the National Health and Nutrition Examination Survey (NHANES) [1] and the Baltimore Longitudinal Study of Aging (BLSA) [2], and clinical studies of chronic disease, such as Alzheimer’s Disease [3], Multiple Sclerosis [4] and Heart Disease [5]. The primary activity measurements in these studies are usually limited to crude summaries of the 24-hour activity cycle such as the total daily activity count, vector magnitude, or number of steps. When walking is of primary scientific interest, steps-based summaries provide useful information about “how much” and “when” the person is walking, but do not provide any information about “how” the person is walking or “whether” their walking changes during the course of the day. This type of information can be crucial in clinical and observational studies as it provides information about the intrinsic characteristics of walking and the associated variability. Understanding the association between these characteristics and levels of fatigue and fatigability in healthy and frail populations is a major step towards identifying parameters that are intuitive, can be easily extracted from raw accelerometry data, and are relevant to health studies. Quantifying gait parameters and ambulatory monitoring of changes in these parameters has become increasingly important for epidemiological, clinical and rehabilitation studies.
Several approaches extracting time-dependent gait parameters were developed and successfully applied to data collected from body-worn accelerometers [6] [7] [8]. These approaches demonstrated a significant discriminative power in studies of clinical pathology [9] [10], fatigability [11] [12], and aging [13] [14] [15]. Stride-to-stride variability is an important gait parameter that quantifies participants’ ability to maintain walking consistency and is strongly associated with motor ability [16] [17] [9]. Stride-to-stride variability has been linked to Mild Cognitive Impairment [18], dementia [19], and stroke [20]. One of the limitations of current approaches is that they are based on data obtained from accelerometers placed around the middle of the body (hip, lower back) [21] [22]. However, more research has recently shifted towards wrist-worn accelerometers such as the Actigraph Link, GENEActiv Watch, Fitbit Flex and Jawbone Up. This shift is likely due to their ease of use, increased compliance of study participants, and improvements in size and battery life [1]. This shift raises new challenges to estimating gait parameters, as hands are involved in a much wider spectrum of activities, which results in higher complexity and increased within- and between-subject variability [23].
We propose a method to extract two measures of stride-to-stride variability: Amplitude Deviation (AD) and Phase Deviation (PD). These measures are based on the amplitude of acceleration and duration of consecutive strides, respectively. We compare the performance of AD and PD calculated based on raw accelerometry data obtained from three body locations: the hip, the left wrist, and the right wrist. We evaluate the sensitivity of AD and PD as a function of on-body placement in 46 participants of the Developmental Epidemiologic Cohort Study [24]. To benchmark AD and PD against standard accelerometry summaries and physical function tests, we evaluate their association with four measures: cadence (C), vector magnitude counts (VMC), time on Five-Times-Sit-To-Stand (Chr5s) test [25] and usual gait speed measured on a 6 meter distance test (Pace6m) [26].
2 Methods
2.1.1 Participants
Eighty-nine community-dwelling older adults were recruited from the Pittsburgh, Pennsylvania area for the National Institute on Aging, Aging Research Evaluating Accelerometry (AREA) project, part of the Developmental Epidemiologic Cohort Study (DECOS). AREA was a methodological initiative designed to examine the impact of accelerometry wear location on assessment of physical activity and sedentary behavior. This report includes data from 46 participants (25 males and 21 females; age: 78±4 y.o.; BMI: 26.75±3) who had completed fast paced 400 meter walk test, were in a good overall physical health and reported no current history of medical conditions that could affect gait. Individuals were excluded from the study if they suffered from any of the following conditions: hip fracture, stroke in the past 12 months, cerebral hemorrhage in the past 6 months; heart attack, angioplasty, or heart surgery in the past 3 months, chest pain during walking in the past 30 days, current treatment for shortness of breath or a lung condition, usual aching, stiffness, or pain in their lower limbs and joints and bilateral difficulty bending or straightening the knees fully [24]. All participants were right-handed.
2.1.2 Measurement protocol
Fast paced 400-meter walk is a standardized test measuring physical function often employed by epidemiological studies [27]. The test consisted of 20 consecutive laps, 20 meters each. For each participant we chose data collected during the second lap of the 400 meters trial as it is expected that during this lap gait parameters are more likely to represent normal gait characteristics for each participant [28]. The reasons are that during the first lap individuals may try to outperform, experience larger variability in the first part of the experiment, while effects of fatigue are less likely to occur only after 20 meters. During the task participants wore three ActiGraph GT3X+ devices (each with three orthogonal axes, sampling frequency: 80 observations/second) located on the hip, the left and right wrists. Data were collected in parallel from all three sensors and synchronized at the sub-second level between devices.
2.2 Data analysis
The three-axial acceleration signal was first reduced to the vector magnitude
which is less sensitive to device rotation and small changes in position. Here x, y, z are the acceleration signals measured along the three orthogonal axes and i represents time. For presentation clarity we have dropped indices corresponding to participant and sensor locations.
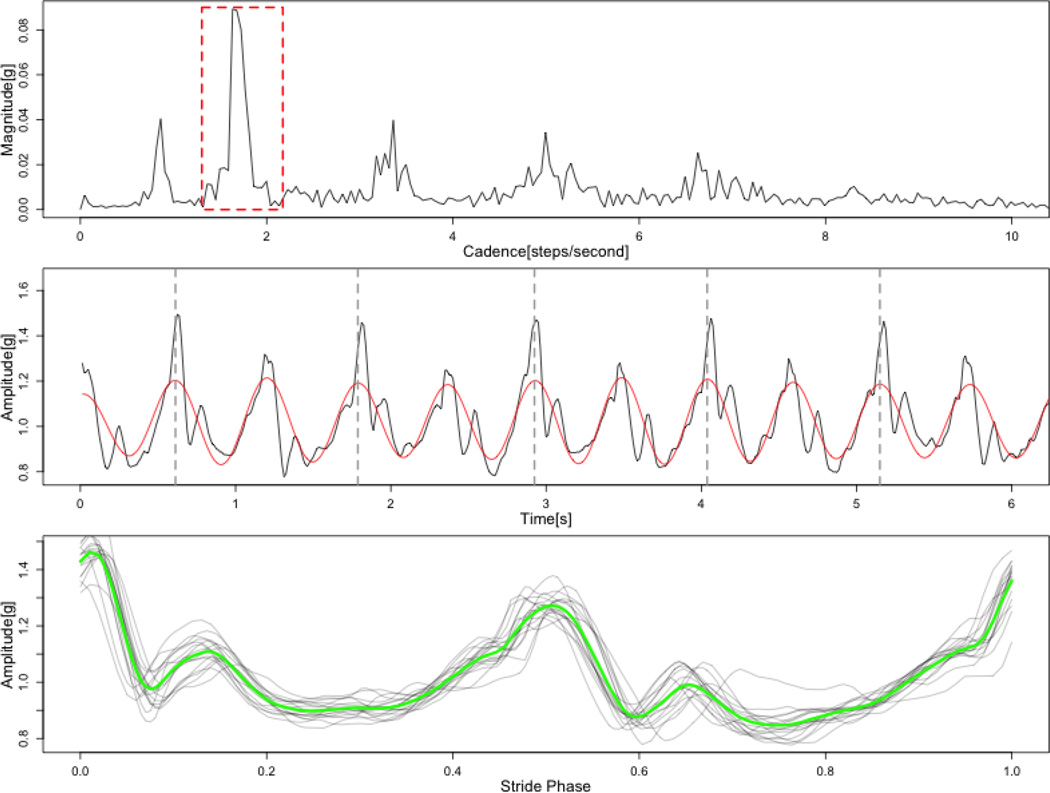
We used fast Fourier transformation of the vector magnitude data from the second lap of the 400-meter walk trial. For each walk-trial and sensor location the mean cadence C was estimated by identifying the spectral peaks corresponding to stride-to-stride frequency. Cadence is expressed in steps-per-second and is defined as C = 2f, where f is the frequency location of the spectral peak corresponding to stride-to-stride frequency (express in Hz). To express cadence in steps per minute, C could be multiplied by 60. An example of Fourier spectrum with estimated location of the peak of interest is presented in the top panel of Figure 1.
Fig.1.
Stages of strides synchronization process. Top panel – Fourier spectrum of gait acceleration signal. Red box marks frequency range corresponding to step-to-step frequency. Middle panel – Time view of gait acceleration signal (black line) and signal after filtration (red line). Bottom panel - synchronized stride profiles (gray lines) and resulting average stride profile (green line).
To estimate the duration of consecutive strides, we first extracted the walking-specific signal using a band-pass FFT filter ranging from 0.75C to 1.25C (where C denotes cadence). We have selected the filter band that depends on cadence to allow for limited variations of the frequency of the extracted signal and, at the same time make sure that neighboring harmonic components of the gait acceleration signal (namely: 0.5C and 1.5 C) will be completely filtered out. The resulting filtered signal can be interpreted as a periodic wave of slowly varying instantaneous frequency that corresponds to durations of consecutive steps. Duration of each stride length was estimated by localizing even zero-crossing points (see fig.2 – middle panel).
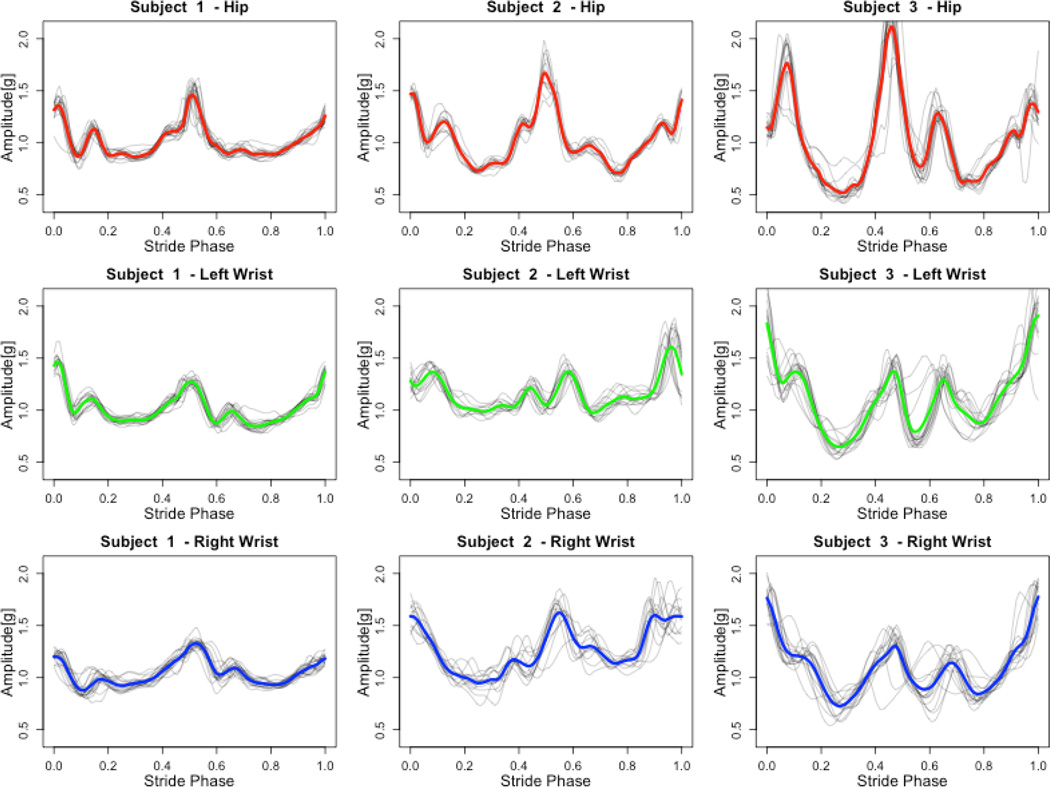
Figure.2.
Exemplary stride profiles for three participants and three sensor locations. Gray lines correspond to registered stride profiles. Color lines represent averaged stride profiles. Red – hip, green – left wrist, blue – right wrist.
2.3 Strides synchronization
After the data transformation steps described in Section 2.2, stride-specific patterns were time-synchronized. To do this, data was first interpolated using splines and then linearly aligned to 0 to 1, where location 0 marks the beginning of the stride cycle while 1 marks its end Fig.1, bottom panel). This transformation allows to estimate the average stride profile for each participant and sensor location. The average value of the amplitude of the acceleration signal within the stride-cycle is expressed as:
where i is an index of a stride phase ranging from 0 to 1, j denotes the stride index, and M is the total number of strides. Examples of average stride acceleration profiles for all three locations are displayed in figure 2.
2.3.1 Stride variability measures
We focus on two measures of stride variability that reflect differences in amplitude and phase. We define the stride-to-stride amplitude deviation (AD) as the mean standard deviation of synchronized activity count profiles:
where N is the number of samples for each synchronized profile, M is the total number of consecutive stride profiles, and Ai denotes the average stride profile at sample time i.
We also define the stride-to-stride phase deviation (PD) as the standard deviation of estimated durations of strides:
where Sj denotes the duration of j-th stride expressed in seconds and 1/f is the average stride length estimated in Section 2.2.
2.3.2 Additional measures
Several additional parameters were estimated. In particular, we estimated the mean cadence, C, for each participant and device location. Cadence was calculated as the inverse of the average stride length multiplied by a factor of two and expressed in steps-per-minute. We have also computed the average vector magnitude count (VMC) for each sensor location. VMC is defined as the mean absolute deviation of the acceleration signal:
where T denotes the total number of samples for each gait acceleration signal.
2.4 Dependent variables
We compare the estimated measures, AD, PD, C and VMC, with performance measures obtained from standardized tests of physical function administered in the DECOS study. We focused on the number of chair stands per second observed during five-times-sit-to-stand (Chr5s) test (mean = 0.4, SD = 0.1) [25] and the usual gait speed measured by a 6-meter walk (Pace6m) test (mean = 1.1, SD = 0.2) [26] and expressed in meters/second.
2.5 Statistical analysis
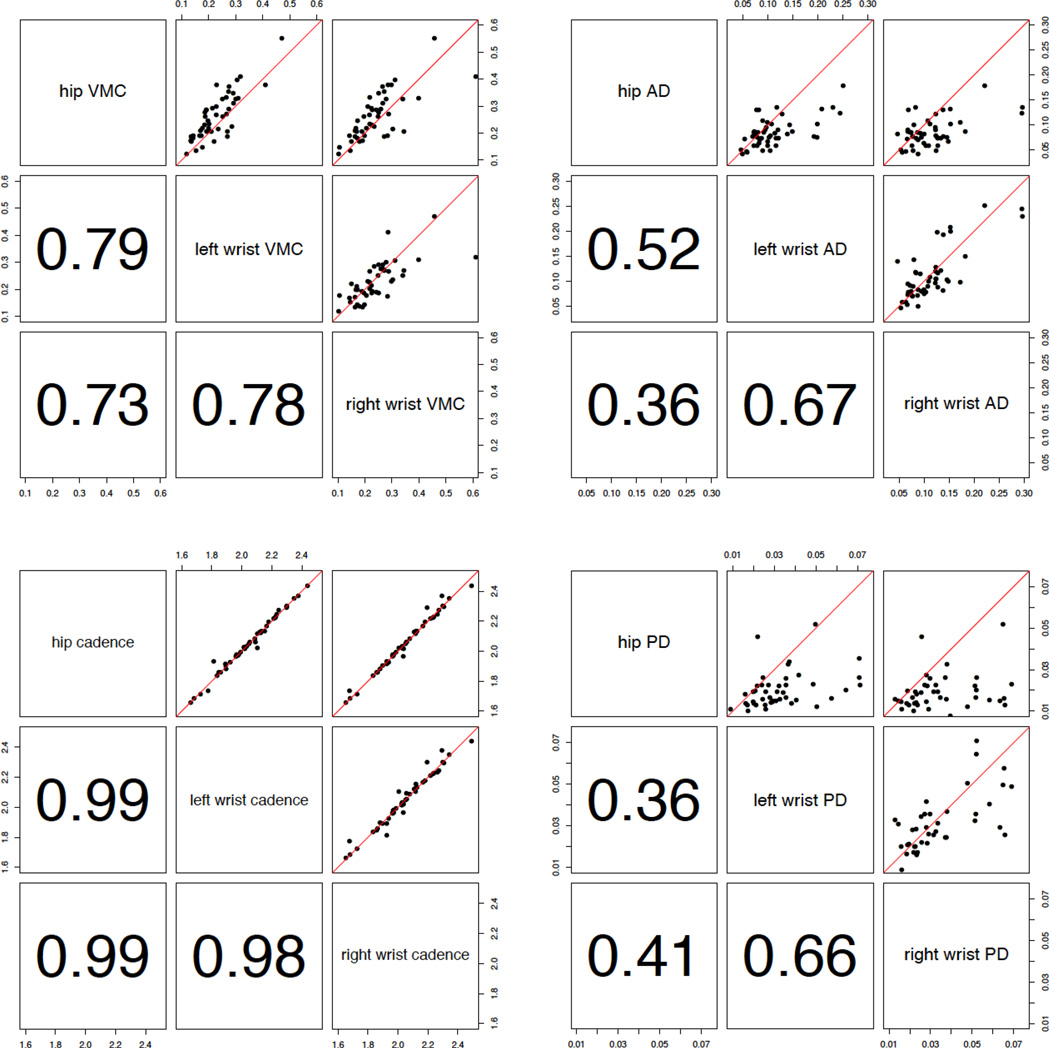
The association between each stride characteristic and dependent variable was evaluated using linear regression models. Each model was adjusted for age. The estimated parameters and p-values for the null hypothesis of no association are presented in table 2. We have also calculated the correlation between measurements using the Spearman’s rank correlation ρ. Figure 3 displays XY-plots of the proposed measures and the estimated correlation coefficients.
Table 2.
Regression coefficients, standard errors and p-values (in brackets) for linear regression fit using proposed values. Shaded fields mark values above significance levels.
| Hip | VMC [g] | AD [g] | Cadence [steps/s] |
PD [s] |
|---|---|---|---|---|
| Chr5s [1/s] | 0.73, 0.140 (<0.001) |
1.54, 0.432 (<0.001) |
0.16, 0.075 (0.043) |
−1.20, 1.585 (0.450) |
| Pace6m [m/s] | −5.15, 1.334 (<0.001) |
−10.20, 4.001 (0.014) |
−1.35, 0.625 (0.036) |
20.00, 13.089 (0.134) |
| Left wrist | VMC [g] | AD [g] | Cadence [steps/s] | PD [s] |
|---|---|---|---|---|
| Chr5s [1/s] | 0.31, 0.096 (0.003) |
0.77, 0.261 (0.005) |
0.13, 0.075 (0.097) |
0.43, 0.450 (0.345) |
| Pace6m [m/s] | −2.01, 0.880 (0.027) |
−2.47, 2.478 (0.325) |
−1.24, 0.630 (0.055) |
1.12, 3.932 (0.777) |
| Right wrist | VMC [g] | AD [g] | Cadence [steps/s] | PD [s] |
|---|---|---|---|---|
| Chr5s [1/s] | 0.25, 0.098 (0.015) |
0.85, 0.242 (0.001) |
0.15, 0.076 (0.058) |
0.65, 0.454 (0.155) |
| Pace6m [m/s] | −1.95, 0.868 (0.030) |
−2.66, 2.371 (0.268) |
−1.37, 0.621 (0.032) |
0.57, 3.496 (0.869) |
Fig.3.
Pair plots displaying proposed measures against each other with red identity lines. Panels below diagonals return Spearman’s rank correlation coefficients.
3 Results
We compared the sensitivity to body location of the four accelerometry-derived gait parameters then studied their association with standard measures of physical function.
3.1 Hip vs. Wrist Placement
Figure 3 provides the pair-wise scatterplots (the upper triangle) and the corresponding Spearman’s rank correlations (the lower triangle) for each of the four gait parameters. Stride-to-stride AD exhibits a relatively high left vs. right wrist correlation (ρ = 0.67), and a lower left-wrist versus hip (ρ = 0.52) and right-wrist versus hip correlations (ρ = 0.36). Stride-to-stride PD indicates higher left versus right wrist correlation (ρ = 0.67), and lower left-wrist versus hip (ρ = 0.36) and right-wrist versus hip correlations (ρ = 0.41). The estimated PD values using wrist accelerometry tend to be larger than PD values obtained using hip accelerometry.
VMC exhibited high correlation between the two wrists (ρ = 0.78) and between the hip and either wrist (ρ = 0.79 and 0.73, respectively). The VMC values estimated from the hip-worn sensor were slightly higher than those obtained from wrist-worn sensors. Cadence indicated much higher correlation across all three locations (ρ = 0.98, 0.99, 0.99) without significant bias.
3.2 Association with physical function tests
Table 2 provides the regression coefficients and the p-values of the tests for the null hypothesis of no-association between the newly proposed measures of gait and standard measures of physical function. Models are adjusted for age and were applied separately for each sensor placement: hip (top panel), left wrist (middle panel), and right wrist (bottom panel). AD was significantly associated with Chr5s for all three locations (p < 0.01) and was significantly associated with Pace6m for the hip-located sensor (p = 0.014). No significant relationship was identified between PD and any of the dependent variables. Walking-related VMC was strongly associated with both Chr5s and Pace6m for all three locations (p < 0.05). Cadence estimated from data collected at the hip was significantly associated with both Chr5s (p = 0.043) and Pace6m (p = 0.037). Cadence estimated from data collected at the right wrist was not statistically associated with Pace6m (p = 0.055) and Chr5s (p = 0.097). We have found statistically significant associations between the cadence estimated from data collected at the left wrist and Pace6m (p = 0.032) but not with Chr5s (p = 0.058).
4 Discussion
We have proposed and examined two measures of stride-to-stride variability, Amplitude Deviation and Phase Deviation, which can be estimated from raw accelerometry data. We demonstrated that AD was significantly associated with the standardized performance tests, Chr5s and Pace6m across three on-body locations including the left and right wrists and the hip. PD was not statistically significantly associated with these performance tests. As our population represents healthy aging participants with no clinical diagnosis, we plan to investigate the performance of PD in specific clinical populations. The mean cadence (C) and the mean vector magnitude count (VMC) were also consistently associated with Chr5s and Pace6m. also compared AD and PD across the three on-body locations, they were more correlated for data collected at the left and the right wrists and less correlated for data collected at the wrist versus hip. This showed that while hip-located sensors produce the most useful data. Wrist however, is still a very promising location, especially for simple yet robust measures like VMC and C.
Presented findings suggest that relatively simple characteristics of stride (VMC and C) and stride-to-stride variability (AD) can be useful to assess the physical function and mobility of individuals. Although, there are existing algorithms can identify fine-tuned parameters of gait [21] [22] and return a wider spectrum of metrics, they often require a symmetric on body placement of the sensor that limits practical applications exclusively to trunk-placed devices. We believe that the proposed measures are more universal and robust across different body locations of the sensor.
One of the limitations of all wrist-based accelerometry-derived gait parameters is that sensors record arm movement, which are proxies of walking strides [29]. Moreover, 400-meter walk does not reflect in full the character of daily walking of individuals. For example, in the free-living environment one would expect long periods of walking without arm swinging (e.g. because hands are in the pocket or handling a phone), walking on different surfaces or climbing stairs. However, our results indicate the accelerometry-derived parameters can be useful in studies of normative aging and may offer opportunities for monitoring temporal gait variability in impaired populations. We conclude that raw accelerometry data contains more detailed information about gait characteristic than the currently used actigraphy summaries.
Our future research will focus on more robust methodology for extraction of gait-parameters that can be implemented to data collected in free-living environment. This is motivated by high demand for objective and unbiased measures of human physical functions as well as growing number of available algorithms for unsupervised segmentation of walking bouts [30] [31].
Table 1.
Means and standard deviations (in brackets) of calculated values
| Hip | Left Wrist | Right Wrist | |
|---|---|---|---|
| VMC [g] | 0.267 (0.093) | 0.245 (0.138) | 0.257 (0.143) |
| AD [g] | 0.086 (0.029) | 0.112 (0.051) | 0.114 (0.053) |
| Cadence [steps/s] | 2.049 (0.181) | 2.049(0.182) | 2.047 (0.183) |
| PD [s] | 0.020 (0.009) | 0.039 (0.029) | 0.046 (0.035) |
Highlights.
Two measures of stride variability based on raw accelerometry data are proposed.
The sensitivity of the approach to accelerometer on-body placement is explored.
Measures have a significant association with the gait speed and sit-to-stand test.
Acknowledgments
Funding
This research was supported by NIH grant RC2AG036594, Pittsburgh Claude D. Pepper Older Americans Independence Center, Research Registry and Developmental Pilot Grant -NIH P30 AG024826 and NIH P30 AG024827 and National Institute on Aging Professional Services Contract HHSN271201100605P. This project was also supported, in part, by the Intramural Research Program of the National Institute on Aging.
This research was supported by the NIH grant RO1 NS085211 from the National Institute of Neurological Disorders and Stroke, by the NIH grant RO1 MH095836 from the National Institute of Mental Health and by NIH grant 1R01 HL123407 from National Hearth, Lung and Blood Institute.
Dr Harezlak’s research was supported in part by the NIMH grant R01MH108467.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
conflict of interest
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- 1.Troiano Richard P, et al. Physical activity in the United States measured by accelerometer. Medicine and science in sports and exercise. Medicine and science in sports and exercise. 2008;40(1):181. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 2.Schrack Jennifer A, et al. Assessing the physical cliff: detailed quantification of age-related differences in daily patterns of physical activity. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013 doi: 10.1093/gerona/glt199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer's disease. Am J Psychiatry. 2001 May;158(5):704–711. doi: 10.1176/appi.ajp.158.5.704. [DOI] [PubMed] [Google Scholar]
- 4.Shammas L, et al. Home-based system for physical activity monitoring in patients with multiple sclerosis (Pilot study) Biomed Eng Online. 2014;13(10) doi: 10.1186/1475-925X-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howell J, et al. Maximum daily 6 minutes of activity: An index of functional capacity derived from actigraphy and its application to older adults with heart failure. Journal of the American Geriatrics Society. 2010;58(5):931–936. doi: 10.1111/j.1532-5415.2010.02805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trojaniello Diana, Cereatti Andrea, Croce Ugo Della. Accuracy, sensitivity and robustness of five different methods for the estimation of gait temporal parameters using a single inertial sensor mounted on the lower trunk. Gait & Posture. 2014;(40):487–492. doi: 10.1016/j.gaitpost.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Yuwono Mitchell, Su Steven W, Guo Ying, Moulton Bruce D, Nguyen Hung T. Unsupervised nonparametric method for gait analysis using awaist-worn inertial sensor. Applied Soft Computing. 2014;(14):72–80. [Google Scholar]
- 8.Zijlstra Wiebren. Assessment of spatio-temporal parameters during unconstrained walking. Eur J Appl Physiol. 2004;(92):39–44. doi: 10.1007/s00421-004-1041-5. [DOI] [PubMed] [Google Scholar]
- 9.Lewek Michael D, Scholz John, Rudolph Katherine S, Snyder-Mackler Lynn. Stride-to-stride variability of knee motion in patients with knee osteoarthritis. Gait & Posture. 2006;(23):505–511. doi: 10.1016/j.gaitpost.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sin S Del, Godfrey A, Rochester L. Validation of an accelerometer to quantify a comprehensive battery of gait characteristics in healthy older adults and Parkinson's disease: toward clinical and at home use. IEEE Journal of Biomedical and Health Informatics. doi: 10.1109/JBHI.2015.2419317. [DOI] [PubMed] [Google Scholar]
- 11.Pruitt LA. Use of accelerometry to measure physical activity in older adults at risk for mobility disability. Journal of aging and physical activity. 16(4):416. doi: 10.1123/japa.16.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy Susan L, Smith Dylan M. Ecological measurement of fatigue and fatigability in older adults with osteoarthritis. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65(2):184–189. doi: 10.1093/gerona/glp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lord Sue, et al. Independent Domains of Gait in Older Adults and Associated Motor and Nonmotor Attributes: Validation of a Factor Analysis Approach. J Gerontol A Biol Sci Med Sci. 2013;68(7):820–827. doi: 10.1093/gerona/gls255. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann Antonia, Luzi Susanna, Murer Kurt, de Bie Rob A, de Bruin Eling D. Concurrent validity of a trunk tri-axial accelerometer system for gait analysis in older adults. Gait & Posture. 2009;(29):444–448. doi: 10.1016/j.gaitpost.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Moe-Nilssen Rolf, Helbostad Jorunn L. Interstride trunk acceleration variability but not step width variability can differentiate between fit and frail older adults. Gait & Posture. 2005;(21):164–170. doi: 10.1016/j.gaitpost.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Zeni Joseph A, Jr, Higginson Jill S. Gait parameters and stride-to-stride variability during familiarization to walking on a split-belt treadmill. Clinical Biomechanics. 2010;(25):383–386. doi: 10.1016/j.clinbiomech.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danion F, Varraine E, Bonnard M, Pailhous J. Stride variability in human gait: the effect of stride frequency and stride length. Gait and Posture. 2003;(18):69–77. doi: 10.1016/s0966-6362(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 18.Beauchet O, Allali G, Launay C, Herrmann FR, Annweiler C. Gait variability at fast-pace walking speed: a biomarker of mild cognitive impairment? The journal of nutrition, health & aging. 2013;17(3):235–239. doi: 10.1007/s12603-012-0394-4. [DOI] [PubMed] [Google Scholar]
- 19.IJmker T, Lamoth CJ. Gait and cognition: the relationship between gait stability and variability with executive function in persons with and without dementia. Gait & posture. 2012;35(1):126–130. doi: 10.1016/j.gaitpost.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 20.iotemporal step characteristics and its relationship to walking performance poststroke. Gait & posture. 2009;29(3):408–414. doi: 10.1016/j.gaitpost.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Mingjing, et al. iGAIT: An interactive accelerometer based gait analysis system. Computer Methods and Programs in Biomedicine. 2012;(108):715–723. doi: 10.1016/j.cmpb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Godfrey A, Din Del S, Barry G, Mathers JC, Rochester L. Instrumenting gaitwith an accelerometer: A system and algorithm examination. Medical Engineering and Physics. 2015;(37):400–407. doi: 10.1016/j.medengphy.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao L, et al. Movement Prediction Using Accelerometers in a Human Population. arXiv preprint arXiv:1404.4601. 2014 doi: 10.1111/biom.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lange-Maia BS. Physical Activity and Change in Long Distance Corridor Walk Performance in the Health, Aging, and Body Composition Study. Journal of the American Geriatrics Society. 2015 Jul;63(7):1348–1354. doi: 10.1111/jgs.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitney Susan L, et al. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test. Physical therapy. 2005;80(10):1034–1045. [PubMed] [Google Scholar]
- 26.Studenski Stephanie, et al. Gait Speed and Survival in Older Adults. Jama. 305(1):50–58. doi: 10.1001/jama.2010.1923. 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. Journal of the American Geriatrics Society. 2001;49(11):1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 28.Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing Fatigability in Mobility-Intact Older Adults. Journal of the American Geriatrics Society. 2014;62(2):347–351. doi: 10.1111/jgs.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyns P, Bruijn SM, Duysens J. The how and why of arm swing during human walking. Gait & posture. 2013;38(4):555–562. doi: 10.1016/j.gaitpost.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Ellis Katherine, Kerr Jacqueline, Godbole Suneeta, Staudenmayer John, Lanckreit Gert. Hip and Wrist Accelerometer Algorithms for Free-Living Behavior Classification. Med Sci Sports Exerc. 2016 May;48(4):933–940. doi: 10.1249/MSS.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbanek Jacek K, et al. Prediction of sustained harmonic walking in the freeliving environment using raw accelerometry data. arXiv preprint arXiv:1505.04066. 2015 doi: 10.1088/1361-6579/aaa74d. [DOI] [PMC free article] [PubMed] [Google Scholar]