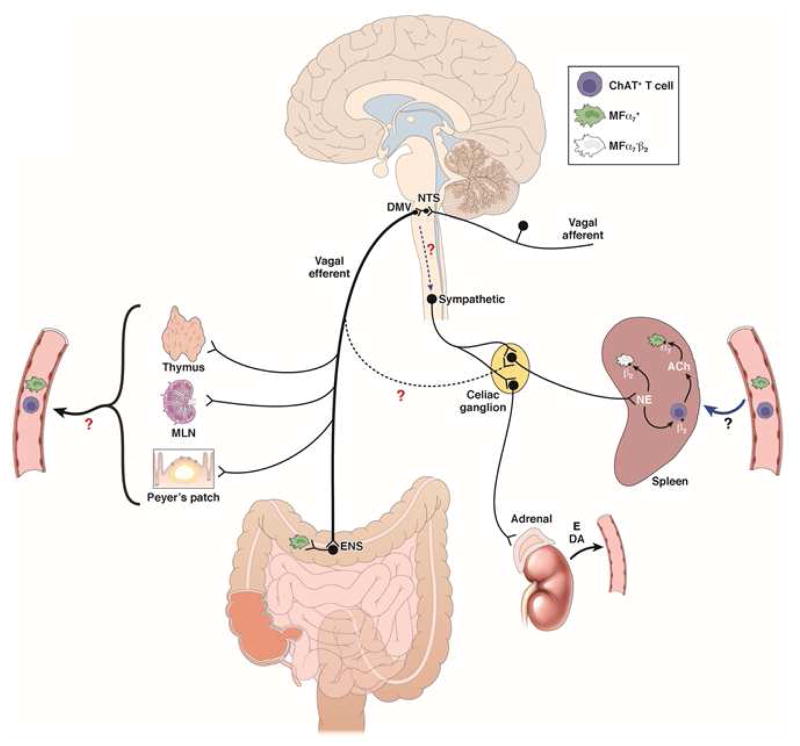
Figure 1. Schematic representation of the cholinergic anti-inflammatory pathway.
VNS of the intact vagus nerve stimulates both afferent and efferent fibers. Electrical stimulation of afferent nerve fibers activates neurons in the NTS leading to activation of not only both ipsi- and contralateral efferent vagus nerves, but also of an adrenergic pathway resulting in release of norepinephrine (NE) in the spleen and the production of dopamine (DA) in the adrenal gland. In the spleen, NE reduces TNF production by splenic macrophages both directly, via actions on β2 adrenoceptor activation, and indirectly, via activation of CHAT+ T cells releasing ACh. Activation of presynaptic α7nAChR on adrenergic nerve fibers156 by choline or other α7nAChR agonists may increase NE release contributing to their anti-inflammatory properties. Stimulation of the efferent vagus nerve dampens α7nAChR+ resident muscular macrophages in the gastrointestinal tract via activation of cholinergic enteric neurons. The immune cell(s) modulated in the lamia propria, however, still need to be identified. The extent to which efferent vagus fibers exert an anti-inflammatory effect in the spleen by synapsing with adrenergic postganglionic (α7nAChR+) neurons in the celiac ganglion is a matter of debate since no anatomical or electrophysiological evidence supporting this connection is available. As the vagus nerve innervates the thymus, Peyers’ patches and other myeloid organs, one may hypothesize that cholinergic modulation of immune cells (α7nAChR+ macrophages, CHAT+, T cells) occurs in these organs. Under conditions of systemic inflammation, these cells subsequently migrate, or get trapped in the spleen, via the circulation.