Abstract
Several of the functions of the human adenovirus type 5 E1B 55 kDa protein are fulfilled via the virus-specific E3 ubiquitin ligase it forms with the viral E4 Orf6 protein and several cellular proteins. Important substrates of this enzyme have not been identified, and other functions, including repression of transcription of interferon-sensitive genes, do not require the ligase. We therefore used immunoaffinity purification and liquid chromatography-mass spectrometry of lysates of normal human cells infected in parallel with HAdV-C5 and E1B 55 kDa protein-null mutant viruses to identify specifically E1B 55 kDa-associated proteins. The resulting set of > 90 E1B-associated proteins contained the great majority identified previously, and was enriched for those associated with the ubiquitin-proteasome system, RNA metabolism and the cell cycle. We also report very severe inhibition of viral genome replication when cells were exposed to both specific or non-specific siRNAs and interferon prior to infection.
Keywords: Human adenovirus type 5 (HAdV-C5), E1B 55 kDa protein, Normal human cells, Mass spectrometry, Type I interferons, Ubiquitin-proteasome system, RNA metabolism
1. Introduction
Successful reproduction of species C human adenoviruses such as HAdV-C5 depends on extensive cooperation among viral and host cell components to achieve efficient expression of viral genes, replication of the viral genome and assembly of progeny virus particles in infected nuclei (Berk, 2013). Various viral gene products also optimize the intracellular milieu by countering cellular responses to infection that would limit virus reproduction. One of several viral proteins that both promote viral gene expression and block anti-viral defenses mounted by the host cell is the E1B 55 kDa protein (Berk, 2013; Blackford and Grand, 2009).
The multi-functional E1B 55 kDa protein assembles with the viral E4 Orf6 protein and the cellular proteins cullin 5, TCEB1, TCEB2 and RBX1 to form a virus-specific E3 ubiquitin ligase (Harada et al., 2002; Querido et al., 2001). The E4 protein interacts with TCEB1 and TCEB2 to recruit the cellular components of this enzyme (Blanchette et al., 2004; Luo et al., 2007), while the E1B protein is thought to serve as the substrate-recognition subunit, as it interacts with such proteins via distinct sequences (Schwartz et al., 2008). These substrates, which are targeted for proteasomal degradation, include the tumor suppressor p53 (Cathomen and Weitzman, 2000; Grand et al., 1994; Harada et al., 2002; Querido et al., 2001, 1997; Roth et al., 1998; Steegenga et al., 1998), the MRE11, RAD50 and NBN proteins that form the MRN complex (Carson et al., 2003; Stracker et al., 2002) and DNA ligase IV (Baker et al., 2007). The p53 protein accumulates to very high concentrations in cells infected by E1B 55 kDa or E4 Orf6 protein-null mutant viruses (Cathomen and Weitzman, 2000; Grand et al., 1994; Moore et al., 1996; Ridgway et al., 1997; Roth et al., 1998; Steegenga et al., 1998; Wienzek et al., 2000), but does not induce apoptosis (Cardoso et al., 2008; Hobom and Dobbelstein, 2004; Miller et al., 2009; O'Shea et al., 2004), as its transcriptional activity is blocked by the viral E4 orf3 protein (Soria et al., 2010) and likely one or more additional viral gene products (DeHart et al., 2015). When the MRN proteins cannot either be degraded in infected cells because of the absence of the E1B 55 kDa or E4Orf6 proteins or relocalized by the E4 Orf3 protein, viral DNA synthesis is severely inhibited (Boyer et al., 1999; Evans and Hearing, 2005; Huang and Hearing, 1989; Lakdawala et al., 2008; Mathew and Bridge, 2007; Shepard and Ornelles, 2004; Stracker et al., 2002). Such inhibition is partly an indirect consequence of concatamerization of viral DNA molecules late in infection (Baker et al., 2007; Boyer et al., 1999; Carson et al., 2003; Lakdawala et al., 2008; Mathew and Bridge, 2007; Weiden and Ginsberg, 1994). However, viral DNA synthesis is also inhibited directly, by a mechanism that is not fully understood but thought to result from sequestration of the viral origins of replication by binding of cellular double-stranded (ds) DNA damage repair proteins (Karen and Hearing, 2011; Karen et al., 2009; Lakdawala et al., 2008; Mathew and Bridge, 2007; Shah and O'Shea, 2015).
Other substrates of the E1B 55 kDa protein-containing E3 ubiquitin ligase that are targeted proteasomal degradation include integrin α3 (Dallaire et al., 2009b), Bloom helicase (Orazio et al., 2011), TIP60 (Gupta et al., 2013), SPOC1 (Schreiner et al., 2013) and ATRX (Berscheminski et al., 2014). The elimination of the lysine acetyl transferase TIP60 relieves repression of E1A gene expression (Gupta, 2013), whereas both SPOC1 and ATRX have been reported to reduce transcription from several viral promoters (Berscheminski et al., 2014; Schreiner et al., 2013). At this juncture, the benefits conferred by degradation of integrin α3 or Bloom helicase have not been established. Conversely, assembly of the virus-specific E3 ubiquitin ligase has been shown to be necessary for an important function of the E1B 55 kDa protein, induction of selective export of viral late mRNAs from the nucleus (Blanchette et al., 2008; Woo and Berk, 2007), but the relevant substrate(s) have not been identified.
Although most of the E1B 55 kDa protein is associated with the E4 Orf6 protein in infected cells (Harada et al., 2002; Sarnow, Sullivan, and Levine, 1982), the E1B protein also possesses E4 Orf6 protein-independent activities. In the absence of other viral proteins, interaction of the E1B 55 kDa protein with p53 greatly stimulates sumoylation of the cellular protein (Muller and Dobner, 2008). Addition of SUMO-1 to p53 also takes place in infected human cell lines, and the E1B 55 kDa protein synthesized in insect cells catalyzes sumoylation of p53 in vitro, indicating that is also an E3 SUMO ligase (Pennella et al., 2010). This modification has been reported to induce colocalization of p53 with the E1B protein in PML-bodies, efficient export of p53 from the nucleus, and inhibition of p53-dependent transcription in cells synthesizing the E1B 55 kDa protein in the absence of other viral proteins (Pennella et al., 2010). The E3 SUMO ligase activity of the E1B protein is therefore thought to make an important contribution to inactivation of p53 in cells transformed by adenoviral E1A and E1B gene products (Muller and Dobner, 2008; Pennella et al., 2010), but how (or whether) it facilitates adenovirus reproduction is not known. A second function ascribed to the E1B 55 kDa protein alone is induction of proteasomal degradation of DAXX (Schreiner et al., 2010). This cellular protein has been reported to interact with the E1B protein (Sieber and Dobner, 2007; Zhao et al., 2003), and is removed from infected cells later in infection than substrates of the virus-specific E3 ubiquitin ligase described above (Schreiner et al., 2010). Such degradation appears to counter inhibition of viral gene expression and replication, as these processes are stimulated to a modest degree when production of DAXX is prevented (Schreiner et al., 2010). The E3 SUMO ligase activity of the E1B 55 kDa protein and its ability to interact with DAXX have also been reported to be necessary for maximal transforming activity of the viral protein (Schreiner et al., 2011).
Another E4 Orf6 protein-independent function of the E1B 55 kD protein is transcriptional repression. This E1B protein is sufficient to block p53-depdendent transcription in vitro and in transient expression assays (Martin and Berk, 1998; Yew and Berk, 1992). Repression of p53-dependent transcription by the E1B protein correlates with the efficiency of transformation of rodent cells in cooperation with E1A gene products (Endter et al., 2005, 2001; Martin and Berk, 1999; Teodoro and Branton, 1997; Teodoro et al., 1994). During the infectious cycle, the E1B 55 kDa protein does not contribute to repression of p53-dependent transcription, as expression of these genes does not increase even when p53 accumulates to high concentrations in infected cells in which the E1B protein is not made (Hobom and Dobbelstein, 2004; Miller et al., 2009; O'Shea et al., 2004; Soria et al., 2010). However, expression of a relatively small set of cellular genes is increased ≥2-fold in normal human cells under these circumstances (Miller et al., 2009). The set of E1B 55 kDa protein-repressed genes is very highly enriched in those associated with immune and anti-viral defenses, with some 40% being interferon-sensitive genes (ISGs). Indeed, we demonstrated that a mutation that prevents production of the E1B 55 kDa protein greatly increases the sensitivity of HAdV-C5 reproduction in normal human cells to type I interferon (IFN), as a result of IFN-induced inhibition of viral DNA synthesis (Chahal et al., 2012). The E1B protein blocks expression of ISGs at the level of transcription, an activity that does not require assembly of the virus-specific E3 ubiquitin ligase (Chahal et al., 2013, 2012). However, substitution of six consecutive residues near the C-terminus of the E1B protein impaired protection against the antiviral action of IFN and repression of ISG transcription (Chahal et al., 2013), consistent with the previous identification of this region as part of the repression domain of the viral protein (Martin and Berk, 1999; Yew et al., 1994). Although the E1B protein has been reported to interact with cellular proteins implicated in transcriptional repression, notably SIN3A (Punga and Akusjarvi, 2000) and DAXX (Schreiner et al., 2010; Sieber and Dobner, 2007; Zhao et al., 2003), the mechanism by which ISG transcription is inhibited is not known.
Previous unbiased efforts to identify E1B 55 kDa-interacting proteins or substrates of the virus E3 ubiquitin ligase have relied on transformed human cell lines as hosts for HAdV-C5 and exploited two dimensional difference gel electrophoresis (Dallaire et al., 2009a, 2009b) or immunoprecipitation and mass spectrometry (MS) (Harada et al., 2002; Querido et al., 2001). The former approach failed to detect known substrates of the virus-specific E3 ubiquitin ligase as proteins that decrease in concentration as the infectious cycle progresses, and hence appears to be limited in sensitivity (Dallaire et al., 2009a). On the other hand, the resolution and sensitivity of MS methods have increased dramatically in the past 15 years (Ahmad and Lamond, 2014; Gallien and Domon, 2015). The studies reported here were undertaken to harness such improvements to identify proteins that associate with the E1B 55 kDa protein in normal human cells.
2. Materials and methods
2.1. Cells and Viruses
Human foreskin fibroblasts (HFFs) were maintained in Dubecco's modified Eagle medium (DMEM, Gibco-ThermoFisher Scientific) containing 7.5% (v/v) bovine growth serum (ThermoFisher Scientific Hyclone). Human 293 (Graham et al., 1977) and adenocarcinoma A549 cells were maintained in DMEM supplemented with 5% (v/v) bovine growth serum and 5% (v/v) calf serum (Gibco-ThermoFisher Scientific).
The phenotypically wild-type derivative of HAdV-C5 AdEasyE1 and the E1B 55 kDa-null mutant virus derived from it, AdEasyE1Δ2347, have been described previously (Kato et al., 2011), as has the substitution mutant AdEasyE1-S19 (Chahal et al., 2013). All viruses were propagated by low multiplicity infection of 293 cells, and virus titers determined by a fluorescent focus assay in which cells were monitored for production of the viral E2 single-stranded DNA-binding protein (E2 DBP). Human 293 cells in monolayer cultures on polylysine-coated 6 well tissue culture plates (ThermoFisher Scientific) were infected in duplicate with 10-fold dilutions of virus stocks for 18 h, when the E2 DBP was visualized by immunofluoresence as described below, and cells stained with DAP1. Fluorescent foci were visualized by fluorescence microscopy using a Nikon TE2000-U inverted microscope. The total number of cells and number of infected cells were counted for 3 random images in each field with at least 200 cells using Image J, and the values were used in conjunction with number of cells/well to calculate virus titer as infectious units/ml.
2.2. Immunoblotting
Viral and cellular proteins were detected by immunoblotting, as described previously (Gonzalez and Flint, 2002), using the anti-E1B 55 kDa protein monoclonal antibody (MAb) 2A6 (Sarnow et al., 1982) the anti-E2 DBP MAb B6 (Reich et al., 1983), the anti-E4 Orf6 protein Mab RSA3 (Marton et al., 1990), the anti-protein V MAb F54 (Lunt et al., 1988) and a polyclonal goat antibody against the cellular protein ANP32A (Santa Cruz Biotechnology). Horse radish peroxidase (HRP)-labelled anti-mouse TruBlot secondary antibodies (eBioScience) and HRP-labelled donkey anti-rabbit antibodies (GE Healthcare) were used to detect mouse and rabbit primary antibodies, respectively, by chemiluminescence detection system with Luminata HRP substrates (Millipore). Cellular β-actin was examined using an HRP-conjugated mouse MAb (Abcam).
2.3. Isolation of the E1B kDa and associated proteins
Pierce protein G-Plus agarose beads (ThermoFisher Scientific) were washed in 0.05 M Tris-HCl, pH 7.5, containing 0.15 M NaCl (TBS) prior to incubation overnight with rotation at 4 °C with 2A6 plus 7F9 mouse anti-E1B 55 kDa protein hybridoma supernatants, or with polyclonal rabbit anti-ANP32A IgG (Bioss). Beads were then washed with TBS and 0.2 M triethanolamine, pH8.9 and incubated for 1 h at room temperature in 0.2 M ethanolamine, pH8.9, containing 0.05 M dimethyl pimelimidate dihydrochloride (cross-linking buffer). They were then washed in 0.2 M triethanolamine, incubated with rocking for 15 min at room temperature with 0.1 M ethanolamine, pH 8.9, washed with 0.2 M triethanolamine, pH12.0, and then with TBS. Protease inhibitors antipain, leupeptin and phenylmethylslfonyl fluoride (PMSF) were added to TBS to final concentrations of 4 µm, 1.7 µm and 1 mM, respectively, and beads stored in this buffer on ice at 4 °C until use.
HFFs were infected with 200 pfu/cell AdEasyE1, AdEasyE1Δ2347 or AdEasyE1-S19 for 24 h., harvested and washed with phosphate-buffered saline (PBS). Cells were suspended in 0.05 M Tris-HCl, pH7.5 containing 0.15 M NaCl 1.5 mM MgCl2, 0.5% (v/v) Non-idet P40 (NP40), 1 mM PMSF and 1 protease inhibitor cocktail tablet (Roche) per 10 ml (NP40 lysis buffer). They were incubated for 5 mins on ice and then with 250 U/ml benzonase (Sigma) for 10 mins at 37 °C. The lysates were sonicated on ice in 10 s bursts until homogenous and centrifuged at 16,000g for 10 mins at 4 °C. Supernatants were precleared by incubation with protein G-Plus agarose beads for 1 h at 4 °C. Anti-E1B 55 kDa IgG beads were added to precleared lysates and the mixtures agitated overnight at 4 °C. Beads were then washed once with NP40 lysis buffer, once with 0.05 M Tris-HCl, pH7.4 containing 0.15 M NaCl, 0.001 M EDTA and 1% (v/v) NP40, twice in the same buffer except containing 0.5 M NaCl, and twice in 0.05 M Tris-HCl, pH7.5 containing 0.01 M MgCl2 and 0.5% (v/v) NP40. For immunoblotting, proteins were released by incubation of beads at 70 °C for 10 mins in 0.12 M Tris-HCl, pH 6.8 containing 4% (w/v) SDS, 20% (v/v) glycerol and 0.004% (w/v) bromphenol blue. For mass spectrometry, proteins were recovered by incubation at 70 °C for 10 mins in 0.12 M Tris-HCl, pH6.8 containing 4% (w/v) SDS and 1 protease inhibitor cocktail tablet per 10 ml.
2.4. Liquid chromatography and mass spectrometry
Proteins recovered from anti-E1B 55 kDa MAb immunoprecipitates were resolved by electrophoresis in 4–12% Novex®-Tris-glycine gels (InVitrogen) and stained with Coomassie blue by standard protocols (Sasse and Gallagher, 2009). Each lane was cut into 6 equal-sized pieces, which were then diced into ~1 mm3 cubes and processed as described previously (DeHart et al., 2014), prior to overnight digestion with trypsin according to the method of Shevchenko et al. (2006). Samples in solution were subjected to buffer exchange into 8 M urea prior to thiol reduction and alkylation and trysin digestion by the FASP procedure (Wisniewski et al., 2009). Samples were desalted as described previously (DeHart et al., 2014) and subjected to reversed-phase nano-LC-MS performed using a Nano Ultra 2D nano-flow capillary high pressure HPLC system (EKsigent) coupled to an LTQ Orbitrap XL hybrid mass spectrometer (ThermoFisher Scientific) outfitted with Triversa NanoMate ion source robot (Advion). Sample concentration, washing and separation were performed as described previously (DeHart et al., 2014), as was acquisition of full scan mass spectra and MS/MS spectra.
The LC-MS/MS data were preprocessed into peaklist files (mgf) using Proteome Discoverer (v 1.3, ThermoFisher Scientific). The files were searched against concatenated Swiss Prot human and species C human adenoviruses databases using the Mascot search engine (v 2.2.7, Matrix Science), allowing for no more than 3 missed trypsin cleavages, carbamidomethylation of cysteines as a fixed modification, and methionine oxidation and N-terminal protein acetylation as variable modifications. Aggregate search results for each sample were imported into scaffold software (Proteome software), consolidated as described previously (DeHart et al., 2014), and filtered to contain 95% protein/90% peptide confidence, requiring 2 peptides per protein assignment, which corresponds to an estimate protein false discovery rate of ≤0.1%.
2.5. Immunofluoresence
Proteins in mock- or infected- cells were visualized by immunofluorescence as described (Gonzalez and Flint, 2002) using the anti-E1B 55 kDa MAb 2A6, the anti-DBP MAb B6, the goat polyclonal anti-ANP32A antibodies listed previously, and a rabbit polyclonal anti-ANP32A antibody (Bioss). Mouse, rabbit and goat primary antibodies were visualized using AlexaFluor®488 chicken anti-mouse IgG, Alexa Fluor®546 goat anti-rabbit IgG, Alexa Fluor®546, and Alexa Flour® 647 donkey anti-goat IgG, respectively, all from InVitrogen.
2.6. Measurement of viral DNA concentrations
Isolation of DNA from mock- or infected HFFs and quantificiation of viral DNA using the ABI PRISM 7900HT sequence detected system were performed as described (Chahal et al., 2012). Viral DNA was detected via an amplicon in the major late (ML) transcription unit (nucleotides 7128–7218 in the HAdV-C5 genome) with the forward and reverse primers described previously (Chahal et al., 2012). An amplicon within the promoter of the human glyceraldehyde-3-phosphate dehydrogenase (GADH) gene was measured to provide an internal control, using the forward primer TAC TAG CGG TTT TAC GGG CG and reverse primer TCG AAC AGG AGG AGC AGA GAG CA. ML values were normalized to the GADPH values and analyzed by the comparative Ct method (Schmittgen and Livak, 2008). All samples were analyzed in triplicate, with at least two experimental replicates. The values shown represent the means of all measurements, and the error bars cumulative standard deviations.
2.7. RNA Interference
HFFs between passages 8 and 13 were grown to 70 – 80% confluence and 10 pmols double-stranded siRNAs introduced using Lipofectamine RNAiMAX (InVitrogen) according to the manufacturer's instructions. The siRNAs, all from Sigma, targeted mRNAs for ANP32A, SIN3A or EGFP, or served as a universal non-targeting control (SIC0001). Two days after siRNA introduction, 250 U/ml type I interferon (PBL Interferon Source) diluted in PBS containing 0.1% (w/ v) bovine serum albumin (BSA) (Sigma-Aldrich) or the BSA solution were added to the medium. After incubation for a further 24 h., cells were infected with 50 pfu/cell and incubated in IFN-containing or control medium for 24 h.
3. Results
3.1. Isolation and identification of proteins associated with the HAdV-C5 E1B 55 kDa protein
Previous ventures to identify E1B 55 kDa protein-interacting proteins relied on the monoclonal antibody 2A6 (Harada et al., 2002; Querido et al., 2001) which recognizes an epitope in the N-terminal segment of the protein (Sarnow et al., 1982); cited in Kao et al., 1990). To avoid failure to capture proteins that interact with the region of the E1B 55 kDa protein containing this epitope, we attempted to construct vectors for expression of tagged E1B proteins in cells infected by mutant viruses defective for synthesis of only this viral protein, such as AdEasyE1Δ2347 (Kato et al., 2012). However, addition of coding sequences specifying, for example, a FLAG tag resulted in plasmid sequence loss in E. coli (N-terminal tag) or prevented the localization of the tagged E1B protein to the nucleus in infected cells (C-terminal tag) (C.J. DeHart and S.J.F. unpublished observations). Consequently, it was necessary to resort to immunoprecipitation of the E1B 55 kDa protein from extracts of HAdV-C5-infected cells.
We had previously purified a (his)6-tagged derivative of the E1B 55 kDa protein from insect cells infected with a baculovirus vector by metal chelate affinity chromatography and gel filtration, and isolated a panel of mouse monoclonal antibodies that recognize the E1B 55 kDa protein in ELISAs (Huang and Flint, 2003). A subset of these monoclonal antibodies of the IgG1 isotype was screened for immunoprecipitation of the E1B 55 kDa protein from HeLa cells infected with the phenotypically wild type virus AdEasyE1 (Kato et al., 2011) for 20 h, and one (7F9) that was efficient as MAb 2A6 identified (data not shown). Immunoaffinity resin to which both 2A6 and 7F9 IgGs were coupled was therefore prepared as described in Materials and Methods, and assessed for immunoprecipitation of the E1B 55 kDa protein from lysates of AdEasy E1-infected HFFs. This protein was readily isolated from such cells, but was not detected in cells infected by the E1B 55 kDa protein null mutant virus AdEasy E1Δ2347 (Fig. 1). The six Ala substitutions (for amino acids 443–448) introduced into the AdEasyE1-S19 E1B 55 kDa protein result in increased sensitivity of viral DNA synthesis to inhibition by type 1 IFN (Chahal et al., 2012), but do not block efficient immunoprecipitation of the protein by the immunoaffinity resin used in these experiments (Fig. 1). As anticipated (see the Introduction), the viral E4 Orf6 protein was also recovered in the immunoprecipitates, but in considerably reduced quantities with the S19 compared to wild type E1B protein (Fig. 1 lower panel). This observation indicates that the S19 substitutions impair the interaction of these viral proteins. As the altered amino acids do not include residues previously reported to be necessary for association of the E1B protein with E4 Orf6 (Rubenwolf et al., 1997; Shen et al., 2001), It seems likely that they alter the conformation of the E1B 55 kDa protein to reduce association with the E4 Orf 6 protein, or the stability of the complex.
Fig. 1.
Recovery of wild-type and altered E1B 55 kDa proteins by immunoprecipitation. HFFs were infected with 200 pfu/cell AdEasyE1, AdEasyE1Δ2347 or AdEasyE1-S19 for 24 h. Whole cell lysates were prepared and incubated with protein G agarose beads to which anti-E1B 55 kDa 2A6 and 7F9 MAbs were cross linked as described in Materials and Methods. The immunoprecipates were washed and eluted with buffer containing 4% (w/v) SDS as described in Materials and Methods. Input (I) and eluted (E1, and E2) proteins (1% and 4% of samples, respectively) were examined by immunoblotting with MAbs specific for the viral E1B 55 kDa (2A6) and E4 Orf6 (RSA-3) proteins.
The immunoaffinity resin carrying the two anti-E1B 55 kDa protein MAbs was exploited to purify the viral and associated proteins from infected normal human cells. HFFs were infected for 24 h with 200 pfu/cell of the phenotypically wild-type virus AdEasyE1 (Kato et al., 2011); the mutant virus AdEasyE1Sub19, which carries substitutions that impair the ability of the E1B 55 kDa protein to block the inhibitory effects of type I interferons (Chahal and Flint, 2012), or, as a control for non-specific binding to the immunoaffinity resin, the E1B 55 kDa null mutant virus AdEasyE1Δ2347 (Chahal and Flint, 2012). Whole cell lysates were prepared and sonicated prior to immunoprecipitation and elution of bound proteins as described in Materials and Methods. Proteins were identified by LC-MS/MS after typsin digestion of the eluted proteins either in solution or after separation by electrophoresis in 4–12% polyacrylamide gels, as described in Materials and Methods. The resulting data were preprocessed into peak list files and searched against the Swiss Prot human and species C adenovirus databases. A total of 845 proteins with at least 2 peptides were identified at a 95% confidence interval in immunoprecipates from AdEasyE1-infected cells. Many of these proteins were also detected in immunoprecipates from HFFs infected with the E1B 55 kDa protein-null mutant virus, and were eliminated from further consideration. The list was filtered further to remove proteins highly represented in MS datasets of negative controls for immunoaffinity purification from human cells of target proteins on agarose beads from the contaminant repository for affinity purification-mass spectrometry database (Mellacheruvu et al., 2013). The final list of 92 E1B 55 kDa protein-associated proteins represents a much larger set than reported previously (Harada et al., 2002; Querido et al., 2001).
The E1B 55 kDa-associated proteins with the greatest numbers of spectral counts (Table 1) include multiple viral and cellular proteins previously reported to interact with the E1B protein. The former group includes the E4 Orf6 protein (see Introduction), as well as the IVa2 and L4 100 K proteins (Harada et al., 2002). The cellular proteins that assemble with the viral E1B 55 kDa and E4 Orf6 proteins to form a virus-specific E3 ubiquitin ligase, as well as its substrates MRE11, NBN and RAD50 were all detected, as were importin α1, NUMAl and ANP32A previously observed in immunoprecipitates of the E1B protein (Harada et al., 2002), and Usp7, which has been reported more recently to bind directly to this viral protein (Ching et al., 2013). The specific recovery of such a substantial number of previously described E1B 55 kDa-interacting proteins affords considerable confidence in the data set described here. Furthermore, this result establishes that cellular proteins listed above associate with the E1B 55 kDa protein in normal human cells as well as in the established cell lines derived from human tumors used in previous studies (Harada et al., 2002; Querido et al., 2001).
Table 1.
Protein immunoprecipitating with E1B 55 kDa proteins
| Protein/gene name | Mol. mass, kDa | Spectral counts |
|
|---|---|---|---|
| AdEasyE1 | AdEasyE1-S19 | ||
| Target | |||
| E1B 55 kDa | 55 | 301 | 281 |
| Viral | |||
| E4 Orf 6 | 37 | 49 | 40 |
| L4 100 kDa | 100 | 69 | 117 |
| pVI | 28 | 7 | 4 |
| pVII | 22 | 3 | 1 |
| Cellular | |||
| CUL5 | 91 | 335 | 165 |
| UBR5 | 309 | 17 | 97 |
| NUMA1 | 237 | 15 | 0 |
| RAD50 | 154 | 13 | 0 |
| ANP32A | 29 | 87 | 49 |
| GGCT | 21 | 8 | 0 |
| TCEB2 | 13 | 38 | 21 |
| USP15 | 112 | 7 | 6 |
| KPNA1 | 60 | 37 | 13 |
| MRE11 | 81 | 22 | 0 |
| TCEB1 | 12 | 83 | 5 |
| FAM111B | 85 | 4 | 6 |
| CEP170 | 161 | 4 | 0 |
| LGALS7 | 15 | 4 | 0 |
| PAPSS2 | 70 | 3 | 2 |
| CASP14 | 28 | 3 | 2 |
| BPTF | 338 | 3 | 0 |
| PIP | 17 | 3 | 0 |
| NBN (NBSI) | 85 | 3 | 0 |
| RNF7 | 13 | 3 | 4 |
| HNRNPR | 71 | 3 | 2 |
| USP7 | 128 | 2 | 3 |
| TRAF7 | 75 | 2 | 4 |
| LASP1 | 30 | 2 | 10 |
| PSMC2 | 48 | 1 | 11 |
| ZNF638 | 221 | 1 | 10 |
| USP34 | 404 | 0 | 38 |
| MYCBP2 | 510 | 0 | 5 |
| NUDC | 38 | 0 | 11 |
| SQSTM1 | 48 | 0 | 3 |
| PSMC6 | 44 | 0 | 9 |
| USP8 | 128 | 16 | 0 |
| THOC2 | 183 | 1 | 2 |
| PSMC4 | 47 | 1 | 2 |
| SRSF3 | 19 | 1 | 1 |
Other reported substrates of the virus-specific E3 ubiquitin ligase, integrin α3 (Dallaire et al., 2009b), DNA Ligase IV (Baker et al., 2007), Bloom helicase (Orazio et al., 2011) and SPOC1 (Schreiner et al., 2013) were not detected. Furthermore, two peptides from the first substrate to be identified, p53 (Harada et al., 2002; Querido et al., 2001) were observed only in immunoprecipates from cells infected by the mutant virus AdEasy E1-S19 (data not shown). The failure to recover these proteins could be because they are targeted for efficient proteasomal degradation by the E1B 55 kDa and E4 Orf6 protein-containing E3 ubiquitin ligase. However, the ready detection of other substrates (Table 1) suggests that this possibility is not very likely. Rather, these other proteins modified by the virus-specific E3 ligase may be present at lower concentrations in the normal human cells used in these experiments than in the tumor-derived cell lines examined previously.
The E1B 55 kDa protein has also been reported to interact with two proteins implicated in regulation of transcription, DAXX (Schreiner et al., 2010; Zhao et al., 2003) and SIN3A (Punga and Akusjarvi, 2000). In a genome-wide RNAi screen, the latter protein was shown to be required for efficient transcription of several interferon-stimulated genes and IFN-mediated inhibition of influenza virus and hepatitis C virus replication (Icardi et al., 2012). Proteins with which DAXX has been reported to associate include regulators of transcription of genes that encode components of the innate immune system (Salomoni and Khelifi, 2006). These proteins were, therefore, of particular interest as possible mediators of E1B 55 kDa protein-dependent repression of ISG transcription. Consequently, we re-analyzed the LC-MS datasets, searching specifically for DAXX and SIN3A peptides. Such peptides were detected, but with similar spectral counts in the protein populations isolated from cells infected by AdEasyE1, AdEasyE1Δ2347 or AdEasyE1-S19 (for example, 3, 3 and 2, respectively for DAXX). Thus, we obtained no evidence for the specific association of either DAXX or SIN3A with viral E1B 55 kDa protein. These discrepanies with previous observation (Punga and Akusjarvi, 2000; Schreiner et al., 2010; Zhao et al., 2003) might indicate that such interactions do not take place in normal human cells, or that they occur later in infection than the very beginning of the late phase examined in these experiments. They may also arise from cell type- specific differences in the production of DAXX and SIN3A or their association with the E1B 55 kDa protein, as the previous studies employed cell lines derived from epithelial cells or hepatocytes, rather fibroblasts as examined in this work.
3.2. Gene ontology analysis of E1B 55 kDa-associated proteins
To obtain an overview of the functions of E1B 55 kDa-interacting proteins, the set of proteins specifically recovered with it in immunoprecipates was assessed for over-representation of gene ontology (GO) terms using the BiNGo Cytoscape plugin (Maere et al., 2005). This analysis identified proteins that participate in a limited number of cellular systems or processes, such as the ubiquitin-proteasome system and RNA metabolism (Table 2). The latter class includes RNA binding proteins, for example several HNRNP proteins, and proteins that participate in pre-mRNA processing reactions. Protein classified in the GO category ubiquitin-dependent catabolic process were also highly enriched. This group contains many of the E1B 55 kDa-associated proteins with the highest spectral counts and includes thiolesterases (e.g. USP7, USP8 and USP47), E3 ubiquitin ligases (e.g. CUL5 and UBR5) and proteins that regulate the cell cycle (Table 2). It is striking that, apart from the cellular components of the virus-specific E3 ubiquitin ligase, previously described E1B 55 kDa-associated proteins also detected in these experiments fall into the cell cycle GO category (Table 2). While the number of these proteins is relatively small, it is possible that their limited functional repertoire reflects the properties of the tumor-derived cells employed as host in previous studies. Be that as it may, the GO analysis reported here suggests that the E1B 55 kDa protein modulates a greater range of host cell processes than previously suspected.
Table 2.
Functional categories of cellular proteins associated with the HAdV-5 E1B 55 kDa protein.
| GO category | P value | Proteins |
|---|---|---|
| Ubiquitin- dependent catabolic process |
 |
NEDD8; SQSTMI; TCEB1; TCEB2; USP15; PSMC6; PSMD4; CUL5; USP34; PSMC2; USP7; PSMC4; UBR5; TRAF7; USP8 |
| Proteolysis involved in cellular protein catabolic process | ||
| Cell Cycle | 6.68 × 10−6 | SMC2; PSMD4; NUDC; p53; NEK9; AP2AI; PIP; MRE11; PSMC6; UBR5; RAD50; PSMC4; NUMA1 |
| RNA Splicing | 1.46 × 10−5 | HNRNPD; THOC2; THOC5; ZFN638; HNRNPAO; SRSF3; AP2A1; SNRBP2; HNRNPR |
3.3. Interactions of the E1B 55 kDa protein impaired by the S19 substitutions
We have reported previously that substitution of Ala for residues 443–448 in the E1B 55 kDa protein leads to impaired repression of interferon sensitive gene transcription and large defects in viral DNA synthesis and production of progeny virus particles in IFN-treated cells infected by the mutant virus AdEasy E1-S19 (Chahal et al., 2013). As the mechanism(s) by which this viral protein counters the IFN-induced anti-viral effects is not yet clear, it was of interest to identify cellular proteins that did not associate efficiently with the S19 E1B 55 kDa protein. Comparison of the spectral counts for proteins immunoprecipitated with the wild-type or S19 E1B proteins defined a small subset absent from or reduced in the S19-E1B 55 kDa-interacting population (Table 3). As the spectral counts for the wild-type and altered E1B 55 kDa proteins were closely similar (Table 3), this difference can be ascribed to impaired association, rather than indirect effects of a decreased concentration of the substituted viral protein. The S19 substitutions did not impair interaction of the E1B 55 kDa protein with the majority of the proteins detected in association with the wild type viral protein, including other viral proteins and the cellular protein USP7, which associates directly with the N-terminal 97 amino acids of the E1B protein (Ching et al., 2013). In contrast, these substitutions eliminated interaction of the E1B 55 kDa protein with all three components of the MRN complex (Table 3), consistent with the previous observation that insertion of 4 amino acids at residue 443 (Yew et al., 1990) prevented degradation of these substrates of the virus-specific E3 ubiquitin ligase (Schwartz et al., 2008). They also prevented association of additional proteins, including NUMA1 and CEP170, and impaired interaction with ANP32A and TCEB1. None of these proteins are known substrates of the E1B 55 kDa- and E4 Orf6 protein-containing E3 ubiquitin ligase, suggesting that they might mediate functions of the E1B protein that do not depend on formation of this enzyme in infected cells. Of this set, ANP32A was chosen for further investigation.
Table 3.
Interactions of the E1B 55 kDa protein impaired by the S19 substitutions
| Protein | Spectral countsa |
Enrichment in WTb | |
|---|---|---|---|
| WT | S19 | ||
| CUL5 | 335 | 165 | 1.9 |
| NUMA1 | 15 | 0 | ≥15 |
| RAD50 | 13 | 0 | ≥13 |
| ANP32A | 87 | 49 | 1.7 |
| MRE11 | 22 | 0 | ≥22 |
| TCEB1 | 83 | 5 | 15.5 |
| CEP170 | 4 | 0 | ≥4 |
| NBN | 3 | 0 | ≥3 |
| E1B 55 kDa | 301 | 281 | NA |
NA = not applicable
Total spectral counts from the in-gel and in-solution MS/MS analyses
The determinate values listed were corrected for the small difference in the abundance of E1B 55 kDa protein spectral counts in the AdEasyE1 and AdEasyE1-S19 immunoprecipitates
The multifunctional ANP32A protein was originally identified as an inhibitor of protein phosphatase 2 A (Li et al., 1996) and has been assigned a variety of physiological functions, including repression of transcription and export of specific mRNAs from the nucleus (see Reilly et al., 2014). In particular, ANP32A has been reported to interact with STAT1 and STAT2 in HeLa cells exposed to IFNβ, and to be required for binding of STAT-containing transcriptional activators to the promoters of ISGs (Kadota and Nagata, 2011). These properties suggested that sequestration of ANP32A by the E1B 55 kDa protein might contribute to the repression of ISG transcription by this viral protein. The ANP32A protein has also been observed to interact with the HAdV-5 E4 Orf6 protein in transformed human and rat cells that also produce viral E1A and E1B gene products (Higashino et al., 2005). In such cells, as well as infected HeLa cells, the E4 Orf6 protein promotes cytoplasmic accumulation and stabilization of short-lived, AU-rich element containing mRNAs, with which this protein, the E1B 55 kDa protein, ANP 32 A and the ARE- and ANP32A-binding protein HuR become associated (Higashino et al., 2005). These interactions switched export of the ARE-containing mRNAs from the Xpo1 export pathway to an Xpo1-independent route (Higashino et al., 2005), raising the possibility that ANP32A might participate in regulation of mRNA export from the nucleus during the late phase of HAdV infection.
3.4. Properties of ANP32A in infected cells
In one approach to validate the association of the E1B 55 kDa protein with ANP32A, we searched the tandem MS/MS spectra of the proteins recovered with the E1B protein for peptides derived from the cellular protein. Ten and 13 such peptides were identified in the protein populations immunoprecipated with the wild type and S19 E1B 55 kDa proteins, respectively, representing 46% and 52% coverage of the ANP32A sequence. The C-terminal 97 residues of ANP32A (some 40% of the total) include a very large number of acidic amino acids and no cleavage sites (after R or K) for trypsin (Fig. 2A). These properties seem likely to account for the absence of peptides from this region of ANP32A in the MS spectra. This cellular protein was also detected readily when immunprecipates of the E1B 55 kDa proteins were examined by immunoblotting (Fig. 2B). Despite similar E1B 55 kDa protein concentration, this protein was recovered in reduced quantities from AdEasy E1-S19-infected cells (Fig. 2B), in agreement with the MS data described previously.
Fig. 2.
Validation of the E1B 55 kDa-ANP32A interaction. A. Peptide coverage of ANP32A immunoprecipitated with the wild type or S19 substituted E1B 55 kDa proteins, with the peptides detected by LC-MS/MS indicated in bold, italic face. These peptides represent 46% and 52%, respectively, of the sequence of ANP32A. B. HFFs were infected with 200 pfu/cell AdEasy E1, AdEasyE1Δ2347 or AdEasyE1-S19 for 24 h., and the E1B 55 kDa protein immunoprecipated from whole cell lysates as described in Materials and Methods. Input (E) and eluted (E1, E2) proteins (1% and 33% of samples, respectively) were examined by immunblotting with the E1B 55 kDa- and ANP32A-specific antibodies described in Materials and Methods.
Before attempting reciprocal co-immunoprecipitation, we examined the steady-state concentration of ANP32A as a function of time after infection of HFFs. In contrast to the substrates of the virus-specific E3 ubiquitin ligase described previously, ANP32A, which appeared as two species perhaps representing phosphorylated and non-phosphorylated forms (Hong et al., 2004; Yu et al., 2004), did not decrease in abundance as the infectious cycle proceded, but rather increased slightly (Fig. 3A). The same response was observed in AdEasy E1Δ2347-infected cells (Fig. 3A), indicating that it does not depend on production of the E1B 55 kDa protein. HFFs were therefore infected with AdEasyE1 for increasing periods, proteins immunoprecipitated from whole cell lysates using anti-ANP32A polyclonal antibodies and the immunoprecipitates examined by immunoblotting with the anti-E1B 55 kDa protein MAB 2A6. As expected, the concentration of the viral protein in the lysates increased considerably as the infectious cycle advanced. A relatively small fraction of the total was recovered with ANP32A at all times examined (Fig. 3B).
Fig. 3.
Effect of HAdV-C5 infection on accumulation of ANP32A: Effect of HAdV-C5 infection on accumulation of ANP32A. HFFs were mock infected (M), or infected with AdEasy E1 or AdEasyE1Δ2347 for the periods indicated and the ANP32A protein examined by immunblotting of whole cell lysates. B. HFFs were mock-infected (O) or infected with 200 pfu/cell AdEasyE1 for the periods indicated. Proteins were immunoprecipitated from whole cell lysates with polyclonal anti-ANP32A antibodies as described in Materials and Methods. Proteins present in lysates (I) and eluted from immnoprecipitates (E) (2% and 10% of samples, respectively were examined by immunoblotting with the 2A6 anti-E1B 55 kDa Mab.
To determine the effect of HAdV-C5 infection, and in particular of the E1B 55 kDa protein, on the intracellular location of ANP32A, HFFs were infected with AdEasyE1 or AdEasyE1Δ2347 for 24 h, or mock-infected. The viral E1B 55 kDa and cellular ANP32A proteins were visualized by indirect immunofluorescence as described in Materials and Methods. The cellular protein was largely nuclear in uninfected HFFs (Fig. 4, panels a–d), as it has also been reported to be in uninfected HeLa cells (Kadota and Nagata, 2011) and human and rat cells transformed by HAdV-C5 E1A and E1B genes (Higashino et al., 2005). ANP32A was also observed to be present in nuclei of wild-type virus-infected cells, but concentrated in discrete dots or foci (Fig. 4, panels e-h). This altered distribution of ANP32A could not be attributed to its interaction with the E1B 55 kDa protein at this time of infection (early in the late phase (Gonzalez et al., 2006): the viral protein appeared as diffuse staining throughout nuclei (Fig. 4, panels e–h), and ANP32A nuclear foci also formed in cells infected by the E1B 55 kDa-null mutant virus AdEasyE1Δ2347 (Fig. 4, panels i–l).
Fig. 4.
Localization of the E1B 55 kDa and ANP32A proteins in infected cells. HFFs were mock-infected or infected with 200 pfu/cell AdEasyE1 or AdEasyEΔ12347 for 24 h, and the viral E1B 55 kDa and cellular ANP32A proteins visualized by immunofluorescence as described in Materials and Methods.
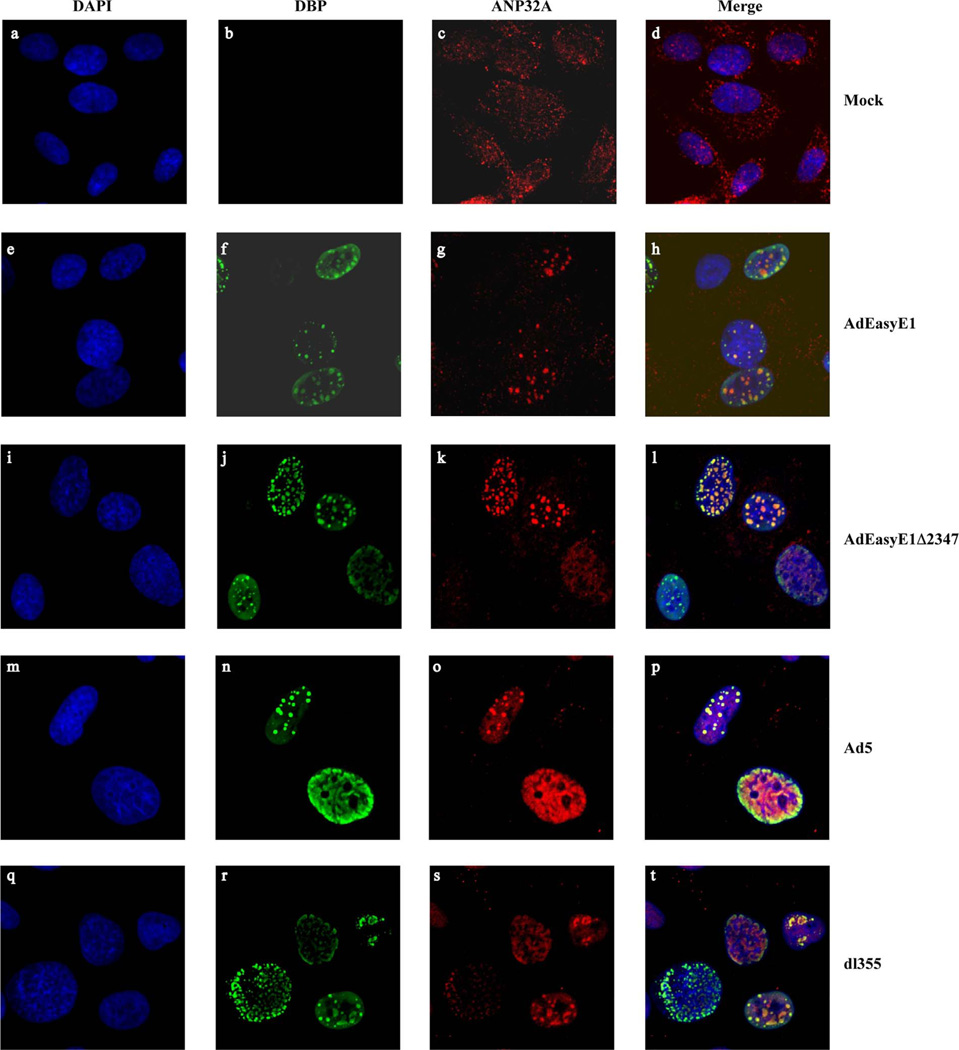
As the punctate appearance of ANP32A in infected cell nuclei was reminiscent of early viral replication centers, we determined whether the cellular protein became associated with these virus-specific structures. Viral replication centers were visualized by virtue of the presence of the E2 single-stranded DNA-binding protein (DBP) (Sugawara et al., 1977; Voelkerding and Klessig, 1986), and were evident as discrete nuclear foci in the majority of HFFs infected by the wild-type or E1B 55 kDa-null mutant viruses (Fig. 5, panels f and j). Some cells displayed diffuse nuclear DBP staining, the pattern observed when replication centers have not yet formed. Most, if not all, of the DBP-containing replication centers were associated with ANP32 in both wild-type (Fig, panels e–h) and E1B 55 kDa null mutant virus–infected cells (Fig. 5, panels i–l). However, in both cases, the cellular protein was also observed in DBP-independent puncta or foci, which might be ANP32A associated with the viral genome and its viral binding protein VII (Xue et al., 2005). In view of the lack of effect of the absence of the E1B 55 kDa protein on the localization of ANP32A and the reported interaction of this cellular protein with the viral E4 Orf6 protein (Higashino et al., 2005), we also examined the distribution of ANP32A in cells infected by the E4 Orf6-null mutant virus dl355 and wild-type HAdV-C5. In both cases, ANP32A was again concentrated at viral replication centers when present and more diffusely distributed when these infected cell-specific structures had not yet formed (Fig. 5, panels m–t). We therefore conclude that neither the E1B 55 kDa nor the E4 Orf6 proteins are necessary for recruitment of ANP32A to viral replication centers.
Fig. 5.
Colocalization of the E2DBP and ANP32A proteins. HAdV-C5 (Ad5) or the mutant dl355 or infected with 200 pfu/ull HFFs were mock-infected as described in the legend to Fig. 4, and the viral E2 DBP and cellular ANP32A proteins examined by immunofluorescence.
3.5. The impact of ANP32A knockdown on viral gene expression
ANP32A has been implicated in export of certain cellular mRNAs from the nucleus (Gallouzi and Steitz, 2001; Gallouzi et al., 2001). Furthermore, its interaction with the HAdV-C5 E4 Orf6 protein has been reported to promote cytoplasmic accumulation of ARE-containing mRNAs via an XPO1-independent pathway (Higashino et al., 2005). The selective export of viral late mRNAs is also XPOl independent (Flint et al., 2005; Yatherajam et al., 2011) and requires the E4 Orf6 protein (Blanchette et al., 2008; Woo and Berk, 2007) raising the possibility that ANP32A might participate in regulation of mRNA export in HAdV-C5-infected cells. We therefore investigated the effect of RNAi-mediated inhibition of ANP32A synthesis on viral late gene expression, by monitoring the accumulation of the late protein, protein V (Berk, 2013), as it is well established that the impaired export of viral late mRNAs in cells infected by E1B 55 kDa-null mutant viruses leads to substantial defects in late protein synthesis (Babiss et al., 1985; Harada and Berk, 1999; Pilder et al., 1986; Williams et al., 1986). We wished to compare the effects of ANP32A knockdown to those resulting from the absence of the E1B 55 kDa protein, but E1B 55 kDa null mutant viruses exhibit impaired viral DNA synthesis in HFFs, and hence delayed progression into the late phase of infection (Chahal and Flint, 2012). Consequently, these experiments were performed in A549 cells.
A small double-stranded interfering RNA (siRNA) specific for ANP32A mRNA or a non-targeting control siRNA was introduced in dividing A549 cells as described in Materials and Methods, and the concentration of the cellular protein examined 3 days thereafter. The control siRNA had no effect on the accumulation of ANP32A, whereas this protein was essentially undetectable in cells that received the ANP32A-specific siRNA (Fig. 6A). Such siRNA-treated cells were infected with 10 pfu/cell AdEasy E1 or AdEasyE1Δ2347 and the accumulation of protein V examined at 24 h. p.i. As anticipated, production of this protein was severely compromised in cells infected by the mutant virus, and knockdown of ANP32A had little further effect (Fig. 6B). Nor did prior knockdown of ANP32A reduce accumulation of protein V in AdEasyE1-infected cells (Fig. 6B), indicating that this cellular protein does not contribute to regulation of viral late mRNA production by the E1B 55 kDa protein.
Fig. 6.
Impact of ANP32A knockdown on viral late gene expression. A. A double-stranded siRNA targeting ANP32A mRNA (ANP) or a universal, non-targeting control siRNA (C) were introduced in A549 cells using lipofectamine RNAiMAX (InVitrogen) as described in Materials and Methods, or were exposed only to lipofectamine (LF) or only to medium (−). Whole cell lysates were prepared 3 days thereafter and ANP32A and β-actin examined by immunoblotting. B. Proliferating A549 cells were treated with ANP32A (ANP) or control (C) siRNAs, or exposed to lipofectamine (LF) or medium (−) only for 72 h. They were then infected with 10 pfu/cell AdEasyE1 or AdeasyE1Δ2347 or mock infected for 24 h. Viral protein V and cellular β-actin in whole cell lysates were examined by immunoblotting.
We also wished to assess the effect of ANP32A knockdown on the sensitivity of viral replication to type I IFN, in this case in HFFs which, in contrast to A549 cells, mount an effective IFN-induced anti-adenoviral defense in the absence of the E1B 55 kDa protein (Chahal et al., 2013). As illustrated in Fig. 7A, introduction of siRNA targeting ANP32A mRNA into proliferating HFFs as described in Materials and Methods, led to a substantial reduction in ANP32A concentration 4 days later, as well as by 3 days later (data not shown), but the non-targeting control siRNAs were without effect. The control or specific siRNA was therefore introduced into HFFs and 250 unit/mol IFN or solvent (PBS plus 0.1% BSA) added to the medium 2 days thereafter. After a further 24 h., the cells were infected, and the concentration of viral DNA measured 24 h. p.i. by quantitative PCR as described in Materials and Methods. We had planned to assess whether the ability of the E1B 55 kDa protein to counter the IFN-induced inhibition of viral genome replication (Chahal and Flint, 2012) was impaired in the absence of ANP32A, and hence to compare IFN-treated cells infected by wild-type and E1B 55 kDa null mutant viruses. However, in initial experiments, we observed that viral DNA synthesis was strongly inhibited in cells exposed to any of three different siRNA prior to AdEasyE1 infection (Fig. 7B). All three siRNAs were introduced into HFFs using lipofectamine RNAiMAX (InVitrogen), raising the possibility that this reagent is deleterious to HFFs and/or viral genome replication. We therefore compared viral DNA synthesis in cells exposed in lipofectamine alone or mock-treated in the absence and presence of type I IFN. The cytokine led to a reduction in viral DNA synthesis of some 12-fold in mock-treated cells (Fig. 7C), a decrease similar in magnitude to those observed in our previous experiments (Chahal et al., 2012). Prior exposure to lipofectamine had no effect on the efficiency of viral DNA synthesis in cells exposed to IFN or vesicle control (Fig. 7C), indicating that the siRNAs induced a strong anti-viral response. This robust and non-specific inhibition of viral genome replication even in cells infected by wild-type virus precluded detection of any responses resulting from the absence of ANP32A.
Fig. 7.
Impact of ANP32A knockdown on viral DNA synthesis in type I IFN-treated cells. A. SiRNAs specific for ANP32A or for EGFP (control, C) were introduced into proliferating HFFs using lipofectamine as described in Materials and Methods, or cells were mock-treated (M). Whole cells lysates were prepared 4 days thereafter and ANP32A and cellular β-actin examined by immunoblotting. B. Proliferating HFFs were treated in siRNAs specific for ANP32A, SIN3A or EGFP (Control) or mock treated as described in Materials and methods, and incubated for 48 h. β-interferon or solvent only was then added to the medium and incubation continued for a further 24 h., when cells were infected with 50 pfu/cell AdEasyE1 or AdEasy E1Δ2347. Total DNA was isolated 24 h. after infection and the concentrations of viral DNA and cellular GAPDH DNA determined by qPCR as described in Materials and Methods. Shown are the ratios of the relative viral DNA concentrations in cells treated with IFN and cells not so treated. C. Viral DNA concentrations were measured as described for panel B in HFFs treated with lipofectamine for 48 h (+LF) or mock treated (−LF) prior to exposure to 250 U/ml type I IFN or solvent for 24 h. followed by infection with 50 pfu/cell AdEasyE1 for 24 h.
4. Discussion
The application of newer methods of collection and analysis of MS data to proteins immunoprecipitated with the HAdV-C5 E1B 55 kDa protein from infected normal human cells has identified an order of magnitude more E1B-associated proteins than described previously (Harada et al., 2002; Querido et al., 2001). This list (Table 1) was filtered to exclude proteins detected in negative control immunoprecipitates of extracts of cells infected by an E1B 55 kDa protein-null mutant virus, as well as proteins routinely contaminating proteomic analyses of samples from human cells in culture (Mellacheruvu et al., 2013). The filtered catalogue includes all subunits of the virus-specific E3 ubiquitin ligase, several of its substrates, all but one of the other viral and cellular proteins previously reported to immunoprecipitate with the E1B 55 kDa protein (Harada et al., 2002), and many additional proteins. The large set of E1B-associated proteins we have identified seems likely to be the result of the greatly improved resolution and sensitivity of MS methods (Ahmed et al., 2014; Gallien and Domon, 2015), but the use of normal human cells as hosts may also be a contributing factor. Infection by HAdV-C5 induces far more limited changes in cellular gene expression in transformed human cells (Zhao et al., 2007) than in normal human cells (Miller et al., 2007; Zhao et al., 2012), especially at the time of infection (soon after entry into the late phase) examined in these experiments, consistent with the view that the protein populations of infected transformed and normal cells are far from identical.
The expanded catalogue of E1B 55 kDa-associated proteins we describe suggests candidiates for cellular proteins that mediate incompletely understood functions of the viral protein, such as regulation of export of mRNA from the nucleus. Selective export of viral late mRNAs requires formation of the E1B 55 kDa protein-containing E3 ubiquitin ligase (Blanchette et al., 2008; Woo and Berk, 2007) and the major cellular mRNA export receptor NXF1 (Yatherajam et al., 2011), but the relevant substrate(s) of the virus-specific ligase have not been identified. The NXF1 receptor is not targeted for proteasomal degradation in HAdV-C5-infected cells, nor are two subunits (THOC1 and THOC4) of the TREX complex (Yatherajam et al., 2011), which couples export to prior reactions in mRNA biogenesis (Katahira, 2012; Reed and Cheng, 2005). One class of proteins enriched in the E1B 55 kDa protein-associated population is defined by the GO term RNA splicing (Table 2). This group includes not only several proteins that mediate or regulate splicing, such as U2 snRNP protein B (SNRBP2), the spliceosome component HNRNPR and the SR proteins SRSF3 and SNF638, but also protein implicated in mRNA export, notably the TREX subunits THOC2 and THOC5. The ser-arg rich protein SRSF3 has also been reported to facilitate export of viral and cellular mRNAs (Escudero-Paunetto et al., 2010; Huang and Steitz, 2001; Shin et al., 2007), by serving as an adaptor for NXF1 and stimulating RNA binding by this export receptor (Muller-McNicoll et al., 2016). It will therefore be of interest to investigate whether any of these proteins is required for selective export of viral late mRNAs, and the impact of HAdV-C5 infection on their concentration, intracellular location and modification state.
Infection of synchronized HeLa cells in S phase with an E1B 55 kDa null mutant virus leads to considerably higher yields of infectious virus particles than does infection of cells in G1 (Goodrum and Ornelles, 1997). The wild type virus can infect all cells in a population regardless of their position in the cell cycle, indicating that the E1B protein is necessary to overcome some restriction on virus reproduction imposed in the G1 phase (Goodrum and Ornelles, 1997). More recently, it has been demonstrated that cells infected by the mutant virus when in G1 phase tend to arrest with the signature of mitotic catastrophe, a response that the E1B 55 kDa protein circumvents by preventing entry into mitosis (Turner et al., 2015). Although there is some evidence that this function of the E1B protein is mediated via effects on p53 (Turner et al., 2015), this viral protein is also associated with a number of other proteins that participate in cell cycle progression and its regulation (Table 2). In addition to the previously identified MRN complex components, this class includes four proteins that mediate or govern mitosis; structural maintenance of choromosomes 2 (SMC2), a subunit of condensin that is important for chromosome condensation prior to mitosis and maintains nuclear architectures (George et al., 2014; Hudson et al., 2003; Saitoh et al., 1995); nuclear migration protein NUDC, which is required for correct spindle formation and cytokinesis (Aumais et al., 2003; Zhang et al., 2002); the serine, threonine protein kinase NEKG (aka NERCC), which is a component of a signaling cascade that governs proper spindle assembly and centrosome separation (Bertran et al., 2011; Roig et al., 2005, 2002), and nuclear mitotic apparatus protein 1 (NUMA1), which tethers microtubules to spindle poles and is important for correct spindle positioning (Kotak and Gonczy, 2013; Radulescu and Cleveland, 2010). Furthermore, several of the E1B 55 kDa – associated proteins that fall into GO categories related to the ubiquitin-proteasome system (Table 2) have been implicated in governing cell cycle progression, and the E3 ubiquitin ligase UDR5 (aka EDD) and the ubiquitin C-terminal hydrolase USF8 (aka ubiquitin isopeptidase Y, UBPY) in regulation of mitosis (Jiang et al., 2015; Mukai et al., 2008; Scialpi et al., 2015). Of particular note, UBR5 is required for maintenance of G2/M arrest in response to dsDNA breaks, and its depletion results in premature entry into mitosis, mitotic catastrophe and cell death (Munoz et al., 2007), phenotypes like those observed in adenovirus-infected cells in which neither the E1B 55 kDa nor the E4 Orf3 protein can be made (Turner et al., 2015). It is therefore possible that one or more of these E1B-associated proteins contributes to the cell cycle-related functions of the E1B 55 kDa protein. Indeed enrichment in the E1B 55 kDa-associated protein population of several proteins that regulate various aspects of cell cycle progression suggests that this viral protein might exert more profound effects on passage of normal human cells through the cycle.
Previous studies have established the potential of the E1B 55 kDa protein to alter the concentrations of host cell proteins by virtue of its function as a subunit of a virus-specific E3 ubiquitin ligase and, independently, as a SUMO E3 ligase (see Introduction). However, proteins of the ubiquitin-proteasome system comprise the two class most enriched in the E1B 55 kDa-associated population (Table 2), suggesting that such potential may be even greater than previously appreciated. These proteins include the E3 ubiquitin C-terminal hydrolyases USP8, 15 and 34 and the ubiquitin ligase regulator NEDD8. Covalent attachment of NEDD8 to cullins is required for maximal ubiquitinylation by cullin-ring ligases (CRLs), including cullins implicated in DNA damage repair responses (Brown and Jackson, 2016), as this modification prevents binding of the CRL inhibitor CAND1 (Soucy et al., 2010; Watson et al., 2011). It is therefore possible that association of NEDD8 with the E1B 55 kDa protein ensures activity of the virus-specific, CUL5-containing E3 ubiquitin ligase, and/or sequesters NEDD8 and reduces ubiquitinylation by other CRLs. Furthermore, addition of NEDD8 to E2F1, a transcriptional activator first identified by virtue of its binding to HAdV-C5 E2 early (ERE) promoter, has been reported to reduce the stability of E2F1 and its transcriptional activity (Loftus et al., 2012). Sequestration of NEDD8 by the E1B 55 kDa protein might therefore contribute to efficient progression through the infectious cycle by preventing loss and inhibition of E2F1, which is required for efficient E2E expression and hence synthesis of viral replication proteins (see Berk, 2013).
Among the various potentially interesting E1B 55 kDa-associated proteins, we chose to focus initially on ANP32A. This protein was also recovered in E1B 55 kDa protein immunopreciptates from HAdV-C5-infected HeLa cells (Harada et al., 2002) and its interaction with the viral protein is impaired by the S19 substitutions (Table 3) that impair repression of ISG transcription (Chahal et al., 2013). Furthermore, as discussed previously, ANP32A has been implicated in both mRNA export from the nucleus and optimal transcription of ISGs. In contrast to substrates of the virus-specific E3 ubiquitin ligase, the steady state concentration of ANP32A increased slightly, rather than decreased, during the late phase of infection (Fig. 3A). Some fraction of this cellular protein was visualized at DBP-containing viral replication centers (Fig. 5). During the late phase of infection in both transformed and normal human cells, a portion of the E1B 55 kDa protein is also localized to viral replication centers and appears in ring-like structures (Gonzalez et al., 2006; Ornelles and Shenk, 1991). However, at the time examined in these experiments (24 h. p.i.), the E1B 55 kDa protein exhibited diffuse nuclear staining (Fig. 4), precluding unambiguous observation of colocalization with ANP32A.
Efficient depletion of ANP32A was achieved using siRNAs in both HeLa cells and HFFs. Despite large reductions in ANP32A concentration in A549 Cells, the viral late protein V accumulated as efficiently as in untreated cells, whereas the absence of the E1B 55 kDa protein, and hence selective export of viral late mRNAs, reduced the concentration of protein V considerably (Fig. 6). We therefore conclude that ANP32A does not make a major contribution to regulation of mRNA export in HAdV-C5-infected cells.
Investigation of responses to the short, interfering RNAs typically used to achieve inhibition of expression of specific genes established that these RNAs activate production of type I IFNs and other cytokines in mammalian cells in culture (Gantier and Williams, 2007; Karpala et al., 2005; Schlee et al., 2006; Sledz et al., 2003) and in mice (Shin et al., 2007). Nevertheless, RNA interference-mediated knockdown of specific genes has been exploited in numerous studies of adenovirus-host cell interactions, with no non-specific, detrimental effects on HAdV-C5 replication. For example, we have exploited this methodology to examine the roles of mRNA export receptor NXF1 (Yatherajam et al., 2011) and the tumor suppressor p53 (Chahal and Flint, 2013) in infected cells, and observed no deleterious responses to control, non-targeting siRNAs. It is also well established that pretreatment of host cells with IFN reduces HAdV-C5 replication to only a modest degree in both transformed and normal human cells (Chahal et al., 2012; Evans and Hearing, 2005; Thimmappaya et al., 1982). However, the results reported here indicate that exposure of cells to both siRNAs (specific or non-specific) and type I IFN prior to infection led to severe inhibition of viral genome replication (Fig. 7). Such inhibition was not attributable to the reagent used to introduce siRNAs, and was an order of magnitude greater than observed in the same type of normal cells treated with only IFN (Chahal et al., 2012). This response precluded any inference about the contribution of ANP32A to the ability of the E1B 55 kDa protein to prevent inhibition of viral DNA synthesis in IFN-treated cells. On the other hand, these observations indicate that, under particular circumstance, HAdV-C5 replication is quite sensitive to the anti-viral defenses induced by IFN, as also reported recently by Hearing and colleagues (Zheng et al., 2016).
Acknowledgments
We thank David Perlman and other staff of the Princeton University Proteomic and Mass Spectrometry Core Facility for invaluable assistance with data collection and analysis. We gratefully acknowledge critical commentary and technical advice provided by Caroline DeHart and Jasdave Chahal. This work was supported by a grant (R56AI091785) from the National Institute of Allergy and Infectious Disease, National Institutes of Health, to S.J. F.
References
- Ahmad Y, Lamond AI. A perspective on proteomics in cell biology. Trends Cell Biol. 2014;24(4):257–264. doi: 10.1016/j.tcb.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W, Gyawali P, Sidhu JP, Toze S. Relative inactivation of faecal indicator bacteria and sewage markers in freshwater and seawater microcosms. Lett. Appl. Microbiol. 2014;59(3):348–354. doi: 10.1111/lam.12285. [DOI] [PubMed] [Google Scholar]
- Aumais JP, Williams SN, Luo W, Nishino M, Caldwell KA, Caldwell GA, Lin SH, Yu-Lee LY. Role for NudC, a dynein-associated nuclear movement protein, in mitosis and cytokinesis. J. Cell. Sci. 2003;116(Pt 10):1991–2003. doi: 10.1242/jcs.00412. [DOI] [PubMed] [Google Scholar]
- Babiss LE, Ginsberg HS, Darnell JE. Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol. Cell. Biol. 1985;5:2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A, Rohleder KJ, Hanakahi LA, Ketner G. Adenovirus E4 34k and E1b 55k oncoproteins target host DNA ligase IV for proteasomal degradation. J. Virol. 2007;81(13):7034–7040. doi: 10.1128/JVI.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk AJ. Adenoviridae. In: Knipe DM, Howley PM, editors. Fields Virology. 6th. Philadelphia, PA: Wolters Kluwer/Lippincott Williams and Wilkins; 2013. [Google Scholar]
- Berscheminski J, Wimmer P, Brun J, Ip WH, Groitl P, Horlacher T, Jaffray E, Hay RT, Dobner T, Schreiner S. Sp100 isoform-specific regulation of human adenovirus 5 gene expression. J. Virol. 2014;88(11):6076–6092. doi: 10.1128/JVI.00469-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran MT, Sdelci S, Regue L, Avruch J, Caelles C, Roig J. Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J. 2011;30(13):2634–2647. doi: 10.1038/emboj.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford AN, Grand RJ. Adenovirus E1B 55-kilodalton protein: multiple roles in viral infection and cell transformation. J. Virol. 2009;83(9):4000–4012. doi: 10.1128/JVI.02417-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette P, Cheng CY, Yan Q, Ketner G, Ornelles DA, Dobner T, Conaway RC, Conaway JW, Branton PE. Both BC-box motifs of adenovirus protein E4orf6 are required to efficiently assemble an E3 ligase complex that degrades p53. Mol. Cell Biol. 2004;24(21):9619–9629. doi: 10.1128/MCB.24.21.9619-9629.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchette P, Kindsmuller K, Groitl P, Dallaire F, Speiseder T, Branton PE, Dobner T. Control of mRNA export by adenovirus E4orf6 and E1B55K proteins during productive infection requires E4orf6 ubiquitin ligase activity. J. Virol. 2008;82(6):2642–2651. doi: 10.1128/JVI.02309-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J, Rohleder K, Ketner G. Adenovirus E4 34k and E4 11k inhibit double strand break repair and are physically associated with the cellular DNA-dependent protein kinase. Virology. 1999;263(2):307–312. doi: 10.1006/viro.1999.9866. [DOI] [PubMed] [Google Scholar]
- Brown JS, Jackson SP. Ubiquitylation, neddylation and the DNA damage response. Open Biol. 2016;5:150018. doi: 10.1098/rsob.150018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso FM, Kato SE, Huang W, Flint SJ, Gonzalez RA. An early function of the adenoviral E1B 55 kDa protein is required for the nuclear relocalization of the cellular p53 protein in adenovirus-infected normal human cells. Virology. 2008;378(2):339–346. doi: 10.1016/j.virol.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Carson CT, Schwartz RA, Stracker TH, Lilley CE, Lee DV, Weitzman MD. The Mre11 complex is required for ATM activation and the G2/M checkpoint. EMBO J. 2003;22(24):6610–6620. doi: 10.1093/emboj/cdg630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathomen T, Weitzman MD. A functional complex of adenovirus proteins E1B-55kDa and E4orf6 is necessary to modulate the expression level of p53 but not its transcriptional activity. J. Virol. 2000;74(23):11407–11412. doi: 10.1128/jvi.74.23.11407-11412.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal JS, Flint SJ. Timely synthesis of the adenovirus type 5 E1B 55- kilodalton protein is required for efficient genome replication in normal human cells. J. Virol. 2012;86(6):3064–3072. doi: 10.1128/JVI.06764-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal JS, Flint SJ. The p53 protein does not facilitate adenovirus type 5 replication in normal human cells. J. Virol. 2013;87(10):6044–6046. doi: 10.1128/JVI.00129-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal JS, Gallagher C, DeHart CJ, Flint SJ. The repression domain of the E1B 55-kilodalton protein participates in countering interferon-induced inhibition of adenovirus replication. J. Virol. 2013;87(8):4432–4444. doi: 10.1128/JVI.03387-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal JS, Qi J, Flint SJ. The human adenovirus type 5 E1B 55 kDa protein obstructs inhibition of viral replication by type I interferon in normal human cells. PLoS Pathog. 2012;8(8):e1002853. doi: 10.1371/journal.ppat.1002853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W, Koyuncu E, Singh S, Arbelo-Roman C, Hartl B, Kremmer E, Speiseder T, Meier C, Dobner T. A ubiquitin-specific protease possesses a decisive role for adenovirus replication and oncogene-mediated transformation. PLoS Pathog. 2013;9(3):e1003273. doi: 10.1371/journal.ppat.1003273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire F, Blanchette P, Branton PE. A proteomic approach to identify candidate substrates of human adenovirus E4orf6-E1B55K and other viral cullin-based E3 ubiquitin ligases. J. Virol. 2009a;83(23):12172–12184. doi: 10.1128/JVI.01169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire F, Blanchette P, Groitl P, Dobner T, Branton PE. Identification of integrin alpha3 as a new substrate of the adenovirus E4orf6/E1B 55-kilodalton E3 ubiquitin ligase complex. J. Virol. 2009b;83(11):5329–5338. doi: 10.1128/JVI.00089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHart CJ, Chahal JS, Flint SJ, Perlman DH. Extensive post-translational modification of active and inactivated forms of endogenous p53. Mol. Cell Proteom. 2014;13(1):1–17. doi: 10.1074/mcp.M113.030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHart CJ, Perlman DH, Flint SJ. Impact of the adenoviral E4 Orf3 protein on the activity and posttranslational modification of p53. J. Virol. 2015;89(6):3209–3220. doi: 10.1128/JVI.03072-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endter C, Hartl B, Spruss T, Hauber J, Dobner T. Blockage of CRM1- dependent nuclear export of the adenovirus type 5 early region 1B 55-kDa protein augments oncogenic transformation of primary rat cells. Oncogene. 2005;24(1):55–64. doi: 10.1038/sj.onc.1208170. [DOI] [PubMed] [Google Scholar]
- Endter C, Kzhyshkowska J, Stauber R, Dobner T. SUMO-1 modification required for transformation by adenovirus type 5 early region 1B 55-kDa oncoprotein. Proc. Natl. Acad. Sci. USA. 2001;98(20):11312–11317. doi: 10.1073/pnas.191361798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero-Paunetto L, Li L, Hernandez FP, Sandri-Goldin RM. SR proteins SRp20 and 9G8 contribute to efficient export of herpes simplex virus 1 mRNAs. Virology. 2010;401(2):155–164. doi: 10.1016/j.virol.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Hearing P. Relocalization of the Mre11-Rad50-Nbs1 complex by the adenovirus E4 ORF3 protein is required for viral replication. J. Virol. 2005;79(10):6207–6215. doi: 10.1128/JVI.79.10.6207-6215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint SJ, Huang W, Goodhouse J, Kyin S. A peptide inhibitor of exportin1 blocks shuttling of the adenoviral E1B 55 kDa protein but not export of viral late mRNAs. Virology. 2005;337:7–17. doi: 10.1016/j.virol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Gallien S, Domon B. Detection and quantification of proteins in clinical samples using high resolution mass spectrometry. Methods. 2015;81:15–23. doi: 10.1016/j.ymeth.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Gallouzi IE, Brennan CM, Steitz JA. Protein ligands of HuR modulate its interaction with target mRNAs in vivo. RNA. 2001;7(9):1348–1361. doi: 10.1083/jcb.151.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallouzi IE, Steitz JA. Delineation of mRNA export pathways by the use of cell-permeable peptides. Science. 2001;294(5548):1841–1842. doi: 10.1126/science.1064693. [DOI] [PubMed] [Google Scholar]
- Gantier MP, Williams BR. The response of mammalian cells to double-stranded RNA. Cytokine Growth Factor Rev. 2007;18(5–6):363–371. doi: 10.1016/j.cytogfr.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CM, Bozler J, Nguyen HQ, Bosco G. Condensins are Required for Maintenance of Nuclear Architecture. Cells. 2014;3(3):865–882. doi: 10.3390/cells3030865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Huang W, Finnen R, Bragg C, Flint SJ. Adenovirus E1B 55-kilodalton protein is required for both regulation of mRNA export and efficient entry into the late phase of infection in normal human fibroblasts. J. Virol. 2006;80(2):964–974. doi: 10.1128/JVI.80.2.964-974.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RA, Flint SJ. Effects of mutations in the adenoviral E1B 55-kilodalton protein coding sequence on viral late mRNA metabolism. J. Virol. 2002;76(9):4507–4519. doi: 10.1128/JVI.76.9.4507-4519.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrum FD, Ornelles DA. The early region 1B 55-kilodalton oncoprotein of adenovirus relieves growth restrictions imposed on viral replication by the cell cycle. J. Virol. 1997;71(1):548–561. doi: 10.1128/jvi.71.1.548-561.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen. Virol. 1977;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Grand RJ, Grant ML, Gallimore PH. Enhanced expression of p53 in human cells infected with mutant adenoviruses. Virology. 1994;203(2):229–240. doi: 10.1006/viro.1994.1480. [DOI] [PubMed] [Google Scholar]
- Gupta A, Jha S, Engel DA, Ornelles DA, Dutta A. Tip60 degradation by adenovirus relieves transcriptional repression of viral transcriptional activator EIA. Oncogene. 2013;32(42):5017–5025. doi: 10.1038/onc.2012.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada JN, Berk AJ. p53-Independent and -dependent requirements for E1B-55K in adenovirus type 5 replication. J. Virol. 1999;73(7):5333–5344. doi: 10.1128/jvi.73.7.5333-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada JN, Shevchenko A, Shevchenko A, Pallas DC, Berk AJ. Analysis of the adenovirus E1B-55K-anchored proteome reveals its link to ubiquitination machinery. J. Virol. 2002;76(18):9194–9206. doi: 10.1128/JVI.76.18.9194-9206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashino F, Aoyagi M, Takahashi A, Ishino M, Taoka M, Isobe T, Kobayashi M, Totsuka Y, Kohgo T, Shindoh M. Adenovirus E4orf6 targets pp32/LANP to control the fate of ARE-containing mRNAs by perturbing the CRM1-dependent mechanism. J. Cell Biol. 2005;170(1):15–20. doi: 10.1083/jcb.200405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobom U, Dobbelstein M. E1B-55-kilodalton protein is not required to block p53-induced transcription during adenovirus infection. J. Virol. 2004;78(14):7685–7697. doi: 10.1128/JVI.78.14.7685-7697.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong R, Macfarlan T, Kutney SN, Seo SB, Mukai Y, Yelin F, Pasternack GR, Chakravarti D. The identification of phosphorylation sites of pp32 and biochemical purification of a cellular pp32-kinase. Biochemistry. 2004;43(31):10157–10165. doi: 10.1021/bi0493968. [DOI] [PubMed] [Google Scholar]
- Huang MM, Hearing P. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J Virol. 1989;63(6):2605–2615. doi: 10.1128/jvi.63.6.2605-2615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Flint SJ. Unusual properties of adenovirus E2E transcription by RNA polymerase III. J. Virol. 2003;77(7):4015–4024. doi: 10.1128/JVI.77.7.4015-4024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Steitz JA. Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol. Cell. 2001;7(4):899–905. doi: 10.1016/s1097-2765(01)00233-7. [DOI] [PubMed] [Google Scholar]
- Hudson DF, Vagnarelli P, Gassmann R, Earnshaw WC. Condensin is required for nonhistone protein assembly and structural integrity of vertebrate mitotic chromosomes. Dev. Cell. 2003;5(2):323–336. doi: 10.1016/s1534-5807(03)00199-0. [DOI] [PubMed] [Google Scholar]
- Icardi L, Mori R, Gesellchen V, Eyckerman S, De Cauwer L, Verhelst J, Vercauteren K, Saelens X, Meuleman P, Leroux-Roels G, De Bosscher K, Boutros M, Tavernier J. The Sin3a repressor complex is a master regulator of STAT transcriptional activity. Proc. Natl. Acad. Sci. USA. 2012;109(30):12058–12063. doi: 10.1073/pnas.1206458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Gomez-Manzano C, Rivera-Molina Y, Lang FF, Conrad CA, Fueyo J. Oncolytic adenovirus research evolution: from cell-cycle checkpoints to immune checkpoints. Curr. Opin. Virol. 2015;13:33–39. doi: 10.1016/j.coviro.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota S, Nagata K. an INHAT component, is a transcription machinery recruiter for maximal induction of IFN-stimulated genes. (pp32) J. Cell Sci. 2011;124(Pt 6):892–839. doi: 10.1242/jcs.078253. [DOI] [PubMed] [Google Scholar]
- Karen KA, Hearing P. Adenovirus core protein VII protects the viral genome from a DNA damage response at early times after infection. J. Virol. 2011;85(9):4135–4142. doi: 10.1128/JVI.02540-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karen KA, Hoey PJ, Young CS, Hearing P. Temporal regulation of the Mre11-Rad50-Nbs1 complex during adenovirus infection. J. Virol. 2009;83(9):4565–4573. doi: 10.1128/JVI.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpala AJ, Doran TJ, Bean AG. Immune responses to dsRNA: implications for gene silencing technologies. Immunol. Cell Biol. 2005;83(3):211–216. doi: 10.1111/j.1440-1711.2005.01331.x. [DOI] [PubMed] [Google Scholar]
- Katahira J. mRNA export and the TREX complex. Biochim. Biophys. Acta. 2012;1819(6):507–513. doi: 10.1016/j.bbagrm.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Kato SE, Chahal JS, Flint SJ. Reduced infectivity of adenovirus type 5 particles and degradation of entering viral genomes associated with incomplete processing of the preterminal protein. J. Virol. 2012;86(24):13554–13565. doi: 10.1128/JVI.02337-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato SE, Huang W, Flint SJ. Role of the RNA recognition motif of the E1B 55 kDa protein in the adenovirus type 5 infectious cycle. Virology. 2011;417(1):9–17. doi: 10.1016/j.virol.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak S, Gonczy P. Mechanisms of spindle positioning: cortical force generators in the limelight. Curr. Opin. Cell Biol. 2013;25(6):741–748. doi: 10.1016/j.ceb.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Lakdawala SS, Schwartz RA, Ferenchak K, Carson CT, McSharry BP, Wilkinson GW, Weitzman MD. Differential requirements of the C terminus of Nbs1 in suppressing adenovirus DNA replication and promoting concatemer formation. J. Virol. 2008;82(17):8362–8372. doi: 10.1128/JVI.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Makkinje A, Damuni Z. Molecular identification of I1PP2A, a novel potent heat-stable inhibitor protein of protein phosphatase 2A. Biochemistry. 1996;35(22):6998–7002. doi: 10.1021/bi960581y. [DOI] [PubMed] [Google Scholar]
- Loftus SJ, Liu G, Carr SM, Munro S, La Thangue NB. NEDDylation regulates E2F-1-dependent transcription. EMBO Rep. 2012;13(9):811–818. doi: 10.1038/embor.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt R, Vayda ME, Young M, Flint SJ. Isolation and characterization of monoclonal antibodies against the adenovirus core proteins. Virology. 1988;164(1):275–279. doi: 10.1016/0042-6822(88)90645-9. [DOI] [PubMed] [Google Scholar]
- Luo K, Ehrlich E, Xiao Z, Zhang W, Ketner G, Yu XF. Adenovirus E4orf6 assembles with Cullin5-ElonginB-ElonginC E3 ubiquitin ligase through an HIV/SIV Vif-like BC-box to regulate p53. FASEB J. 2007;21(8):1742–1750. doi: 10.1096/fj.06-7241com. [DOI] [PubMed] [Google Scholar]
- Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21(16):3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- Martin ME, Berk AJ. Adenovirus E1B 55K represses p53 activation in vitro. J. Virol. 1998;72(4):3146–3154. doi: 10.1128/jvi.72.4.3146-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ME, Berk AJ. Corepressor required for adenovirus E1B 55,000- molecular-weight protein repression of basal transcription. Mol. Cell Biol. 1999;19(5):3403–3414. doi: 10.1128/mcb.19.5.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton MJ, Baim SB, Ornelles DA, Shenk T. The adenovirus E4 17-kilodalton protein complexes with the cellular transcription factor E2F, altering its DNA-binding properties and stimulating E1A-independent accumulation of E2 mRNA. J. Virol. 1990;64(5):2345–2359. doi: 10.1128/jvi.64.5.2345-2359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SS, Bridge E. The cellular Mre11 protein interferes with adenovirus E4 mutant DNA replication. Virology. 2007;365(2):346–355. doi: 10.1016/j.virol.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Mellacheruvu D, Wright Z, Couzens AL, Lambert JP, St-Denis NA, Li T, Miteva YV, Hauri S, Sardiu ME, Low TY, Halim VA, Bagshaw RD, Hubner NC, Al-Hakim A, Bouchard A, Faubert D, Fermin D, Dunham WH, Goudreault M, Lin ZY, Badillo BG, Pawson T, Durocher D, Coulombe B, Aebersold R, Superti-Furga G, Colinge J, Heck AJ, Choi H, Gstaiger M, Mohammed S, Cristea IM, Bennett KL, Washburn MP, Raught B, Ewing RM, Gingras AC, Nesvizhskii AI. The CRAPome: a contaminant repository for affinity purification-mass spectrometry data. Nat. Methods. 2013;10(8):730–736. doi: 10.1038/nmeth.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Myers CL, Rickards B, Coller HA, Flint SJ. Adenovirus type 5 exerts genome-wide control over cellular programs governing proliferation, quiescence, and survival. Genome Biol. 2007;8(4):R58. doi: 10.1186/gb-2007-8-4-r58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL, Rickards B, Mashiba M, Huang W, Flint SJ. The adenoviral E1B 55-kilodalton protein controls expression of immune response genes but not p53- dependent transcription. J. Virol. 2009;83(8):3591–3603. doi: 10.1128/JVI.02269-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M, Horikoshi N, Shenk T. Oncogenic potential of the adenovirus E4orf6 protein. Proc. Natl. Acad. Sci. USA. 1996;93(21):11295–11301. doi: 10.1073/pnas.93.21.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai A, Mizuno E, Kobayashi K, Matsumoto M, Nakayama KI, Kitamura N, Komada M. Dynamic regulation of ubiquitylation and deubiquitylation at the central spindle during cytokinesis. J. Cell Sci. 2008;121(Pt 8):1325–1333. doi: 10.1242/jcs.027417. [DOI] [PubMed] [Google Scholar]
- Muller-McNicoll M, Botti V, de Jesus Domingues AM, Brandl H, Schwich OD, Steiner MC, Curk T, Poser I, Zarnack K, Neugebauer KM. SR proteins are NXF1 adaptors that link alternative RNA processing to mRNA export. Genes Dev. 2016;30(5):553–566. doi: 10.1101/gad.276477.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Dobner T. The adenovirus E1B-55K oncoprotein induces SUMO modification of p53. Cell Cycle. 2008;7(6):754–758. doi: 10.4161/cc.7.6.5495. [DOI] [PubMed] [Google Scholar]
- Munoz MA, Saunders DN, Henderson MJ, Clancy JL, Russell AJ, Lehrbach G, Musgrove EA, Watts CK, Sutherland RL. The E3 ubiquitin ligase EDD regulates S-phase and G(2)/M DNA damage checkpoints. Cell Cycle. 2007;6(24):3070–3077. doi: 10.4161/cc.6.24.5021. [DOI] [PubMed] [Google Scholar]
- O'Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, Boyle L, Pandey K, Soria C, Kunich J, Shen Y, Habets G, Ginzinger D, McCormick F. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6(6):611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Orazio NI, Naeger CM, Karlseder J, Weitzman MD. The adenovirus E1b55K/E4orf6 complex induces degradation of the Bloom helicase during infection. J. Virol. 2011;85(4):1887–1892. doi: 10.1128/JVI.02134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelles DA, Shenk T. Localization of the adenovirus early region 1B 55- kilodalton protein during lytic infection: association with nuclear viral inclusions requires the early region 4 34-kilodalton protein. J. Virol. 1991;65(1):424–429. doi: 10.1128/jvi.65.1.424-429.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennella MA, Liu Y, Woo JL, Kim CA, Berk AJ. Adenovirus E1B 55-kilodalton protein is a p53-SUMO1 E3 ligase that represses p53 and stimulates its nuclear export through interactions with promyelocytic leukemia nuclear bodies. J. Virol. 2010;84(23):12210–12225. doi: 10.1128/JVI.01442-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilder S, Moore M, Logan J, Shenk T. The adenovirus E1B-55kd transforming polypeptide modulates transport or cytoplasmic stablization of viral and host cell mRNAs. Mol. Cell. Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punga T, Akusjarvi G. The adenovirus-2 E1B-55K protein interacts with a mSin3A/histone deacetylase 1 complex. FEBS Lett. 2000;476(3):248–252. doi: 10.1016/s0014-5793(00)01739-7. [DOI] [PubMed] [Google Scholar]
- Querido E, Morrison MR, Chu-Pham-Dang H, Thirlwell SW, Boivin D, Branton PE. Identification of three functions of the adenovirus e4orf6 protein that mediate p53 degradation by the E4orf6-E1B55K complex. J. Virol. 2001;75(2):699–709. doi: 10.1128/JVI.75.2.699-709.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querido E, Teodoro JG, Branton PE. Accumulation of p53 induced by the adenovirus E1A protein requires regions involved in the stimulation of DNA synthesis. J. Virol. 1997;71(5):3526–3533. doi: 10.1128/jvi.71.5.3526-3533.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulescu AE, Cleveland DW. NuMA after 30 years: the matrix revisited. Trends Cell Biol. 2010;20(4):214–222. doi: 10.1016/j.tcb.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R, Cheng H. TREX, SR proteins and export of mRNA. Curr. Opin. Cell Biol. 2005;17(3):269–273. doi: 10.1016/j.ceb.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Reich NC, Oren M, Levine AJ. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol. Cell Biol. 1983;3(12):2143–2150. doi: 10.1128/mcb.3.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly PT, Yu Y, Hamiche A, Wang L. Cracking the ANP32 whips: important functions, unequal requirements and hints at disease implication. Bioessays. 2014;36(11):1062–1073. doi: 10.1002/bies.201400058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway PJ, Hall AR, Myers CJ, Braithwaite AW. p53/E1b58kDa complex regulates adenovirus replication. Virology. 1997;237(2):404–413. doi: 10.1006/viro.1997.8782. [DOI] [PubMed] [Google Scholar]
- Roig J, Groen A, Caldwell J, Avruch J. Active Nercc1 protein kinase concentrates at centrosomes early in mitosis and is necessary for proper spindle assembly. Mol. Biol. Cell. 2005;16(10):4827–4840. doi: 10.1091/mbc.E05-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roig J, Mikhailov A, Belham C, Avruch J. Nercc1, a mammalian NIMA-family kinase, binds the Ran GTPase and regulates mitotic progression. Genes Dev. 2002;16(13):1640–1658. doi: 10.1101/gad.972202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J, Konig C, Wienzek S, Weigel S, Ristea S, Dobbelstein M. Inactivation of p53 but not p73 by adenovirus type 5 E1B 55-kilodalton and E4 34-kilodalton oncoproteins. J. Virol. 1998;72(11):8510–8516. doi: 10.1128/jvi.72.11.8510-8516.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenwolf S, Schutt H, Nevels M, Wolf H, Dobner T. Structural analysis of the adenovirus type 5 E1B 55-kilodalton-E4orf6 protein complex. J. Virol. 1997;71(2):1115–1123. doi: 10.1128/jvi.71.2.1115-1123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh N, Goldberg I, Earnshaw WC. The SMC proteins and the coming of age of the chromosome scaffold hypothesis. Bioessays. 1995;17(9):759–766. doi: 10.1002/bies.950170905. [DOI] [PubMed] [Google Scholar]
- Salomoni P, Khelifi AF. Daxx: death or survival protein? Trends Cell Biol. 2006;16(2):97–104. doi: 10.1016/j.tcb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Sarnow P, Sullivan CA, Levine AJ. A monoclonal antibody detecting the Ad5 E1B-58K tumor antigen in adenovirus-infected and transformed cells. Virology. 1982;120:387–394. doi: 10.1016/0042-6822(82)90054-x. [DOI] [PubMed] [Google Scholar]
- Sasse J, Gallagher SR. Staining proteins in gels. Curr Protoc Mol Biol. 2009 doi: 10.1002/0471142727.mb1006s85. Chapter 10, Unit 10 6. [DOI] [PubMed] [Google Scholar]
- Schlee M, Hornung V, Hartmann G. siRNA and isRNA: two edges of one sword. Mol. Ther. 2006;14(4):463–470. doi: 10.1016/j.ymthe.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schreiner S, Kinkley S, Burck C, Mund A, Wimmer P, Schubert T, Groitl P, Will H, Dobner T. SPOC1-mediated antiviral host cell response is antagonized early in human adenovirus type 5 infection. PLoS Pathog. 2013;9(11):e1003775. doi: 10.1371/journal.ppat.1003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner S, Wimmer P, Groitl P, Chen SY, Blanchette P, Branton PE, Dobner T. Adenovirus type 5 early region 1B 55K oncoprotein-dependent degradation of cellular factor Daxx is required for efficient transformation of primary rodent cells. J. Virol. 2011;85(17):8752–8765. doi: 10.1128/JVI.00440-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner S, Wimmer P, Sirma H, Everett RD, Blanchette P, Groitl P, Dobner T. Proteasome-dependent degradation of Daxx by the viral E1B-55K protein in human adenovirus-infected cells. J. Virol. 2010;84(14):7029–7038. doi: 10.1128/JVI.00074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz RA, Lakdawala SS, Eshleman HD, Russell MR, Carson CT, Weitzman MD. Distinct requirements of adenovirus E1b55K protein for degradation of cellular substrates. J. Virol. 2008;82(18):9043–9055. doi: 10.1128/JVI.00925-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialpi F, Mellis D, Ditzel M. EDD, a ubiquitin-protein ligase of the N-end rule pathway, associates with spindle assembly checkpoint components and regulates the mitotic response to nocodazole. J. Biol. Chem. 2015;290(20):12585–12594. doi: 10.1074/jbc.M114.625673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah GA, O'Shea CC. Viral and Cellular Genomes Activate Distinct DNA Damage Responses. Cell. 2015;162(5):987–1002. doi: 10.1016/j.cell.2015.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Kitzes G, Nye JA, Fattaey A, Hermiston T. Analyses of single-amino-acid substitution mutants of adenovirus type 5 E1B-55K protein. J. Virol. 2001;75(9):4297–4307. doi: 10.1128/JVI.75.9.4297-4307.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard RN, Ornelles DA. Diverse roles for E4orf3 at late times of infection revealed in an E1B 55-kilodalton protein mutant background. J. Virol. 2004;78(18):9924–9935. doi: 10.1128/JVI.78.18.9924-9935.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006;1(6):2856–2860. doi: 10.1038/nprot.2006.468. [DOI] [PubMed] [Google Scholar]
- Shin D, Kim SI, Park M, Kim M. Immunostimulatory properties and antiviral activity of modified HBV-specific siRNAs. Biochem. Biophys. Res. Commun. 2007;364(3):436–442. doi: 10.1016/j.bbrc.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Sieber T, Dobner T. Adenovirus type 5 early region 1B 156R protein promotes cell transformation independently of repression of p53-stimulated transcription. J. Virol. 2007;81(1):95–105. doi: 10.1128/JVI.01608-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BR. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 2003;5(9):834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- Soria C, Estermann FE, Espantman KC, O'Shea CC. Heterochromatin silencing of p53 target genes by a small viral protein. Nature. 2010;466(7310):1076–1081. doi: 10.1038/nature09307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy TA, Dick LR, Smith PG, Milhollen MA, Brownell JE. The NEDD8 Conjugation Pathway and Its Relevance in Cancer Biology and Therapy. Genes Cancer. 2010;1(7):708–716. doi: 10.1177/1947601910382898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegenga WT, Riteco N, Jochemsen AG, Fallaux FJ, Bos JL. The large E1B protein together with the E4orf6 protein target p53 for active degradation in adenovirus infected cells. Oncogene. 1998;16(3):349–357. doi: 10.1038/sj.onc.1201540. [DOI] [PubMed] [Google Scholar]
- Stracker TH, Carson CT, Weitzman MD. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature. 2002;418(6895):348–352. doi: 10.1038/nature00863. [DOI] [PubMed] [Google Scholar]
- Sugawara K, Gilead Z, Wold WS, Green M. Immunofluorescence study of the adenovirus type 2 single-stranded DNA binding protein in infected and transformed cells. J. Virol. 1977;22(2):527–539. doi: 10.1128/jvi.22.2.527-539.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro JG, Branton PE. Regulation of p53-dependent apoptosis, transcriptional repression, and cell transformation by phosphorylation of the 55- kilodalton E1B protein of human adenovirus type 5. J. Virol. 1997;71(5):3620–3627. doi: 10.1128/jvi.71.5.3620-3627.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro JG, Halliday T, Whalen SG, Takayesu D, Graham FL, Branton PE. Phosphorylation at the carboxy terminus of the 55-kilodalton adenovirus type 5 E1B protein regulates transforming activity. J. Virol. 1994;68(2):776–786. doi: 10.1128/jvi.68.2.776-786.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B, Weinberger C, Schneider RJ, Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Turner RL, Groitl P, Dobner T, Ornelles DA. Adenovirus replaces mitotic checkpoint controls. J. Virol. 2015;89(9):5083–5096. doi: 10.1128/JVI.00213-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkerding K, Klessig DF. Identification of two nuclear subclasses of the adenovirus type 5- encoded DNA-binding protein. J. Virol. 1986;60(2):353–362. doi: 10.1128/jvi.60.2.353-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson IR, Irwin MS, Ohh M. NEDD8 pathways in cancer, Sine Quibus Non. Cancer Cell. 2011;19(2):168–176. doi: 10.1016/j.ccr.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Weiden MD, Ginsberg HS. Deletion of the E4 region of the genome produces adenovirus DNA concatemers. Proc. Natl. Acad. Sci. USA. 1994;91(1):153–157. doi: 10.1073/pnas.91.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienzek S, Roth J, Dobbelstein M. E1B 55-kilodalton oncoproteins of adenovirus types 5 and 12 inactivate and relocalize p53, but not p51 or p73, and cooperate with E4orf6 proteins to destabilize p53. J. Virol. 2000;74(1):193–202. doi: 10.1128/jvi.74.1.193-202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J, Karger BD, Ho YS, Castiglia CL, Mann T, Flint SJ. The adenovirus E1B 495R protein plays a role in regulating the transport and stability of the viral late messages. Cancer. Cells. 1986;4:275–284. [Google Scholar]
- Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat. Methods. 2009;6(5):359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- Woo JL, Berk AJ. Adenovirus ubiquitin-protein ligase stimulates viral late mRNA nuclear export. J. Virol. 2007;81(2):575–587. doi: 10.1128/JVI.01725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Johnson JS, Ornelles DA, Lieberman J, Engel DA. Adenovirus protein VII functions throughout early phase and interacts with cellular proteins SET and pp32. J. Virol. 2005;79(4):2474–2483. doi: 10.1128/JVI.79.4.2474-2483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatherajam G, Huang W, Flint SJ. Export of adenoviral late mRNA from the nucleus requires the Nxf1/Tap export receptor. J. Virol. 2011;85(4):1429–1438. doi: 10.1128/JVI.02108-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew PR, Berk AJ. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992;357(6373):82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- Yew PR, Kao CC, Berk AJ. Dissection of functional domains in the adenovirus 2 early 1B 55k polypeptide by suppressor-linker-insertional mutagenesis. Virol. 1990;179:795–805. doi: 10.1016/0042-6822(90)90147-j. [DOI] [PubMed] [Google Scholar]
- Yew PR, Liu X, Berk AJ. Adenovirus E1B oncoprotein tethers a transcriptional repression domain to p53. Genes Dev. 1994;8(2):190–202. doi: 10.1101/gad.8.2.190. [DOI] [PubMed] [Google Scholar]
- Yu LG, Packman LC, Weldon M, Hamlett J, Rhodes JM. Protein phosphatase 2A, a negative regulator of the ERK signaling pathway, is activated by tyrosine phosphorylation of putative HLA class II-associated protein I (PHAPI)/ pp32 in response to the antiproliferative lectin, jacalin. J. Biol. Chem. 2004;279(40):41377–41383. doi: 10.1074/jbc.M400017200. [DOI] [PubMed] [Google Scholar]
- Zhang MY, Huang NN, Clawson GA, Osmani SA, Pan W, Xin P, Razzaque MS, Miller BA. Involvement of the fungal nuclear migration gene nudC human homolog in cell proliferation and mitotic spindle formation. Exp. Cell Res. 2002;273(1):73–84. doi: 10.1006/excr.2001.5414. [DOI] [PubMed] [Google Scholar]
- Zhao B, Hou S, Ricciardi RP. Chromatin repression by COUP-TFII and HDAC dominates activation by NF-kappaB in regulating major histocompatibility complex class I transcription in adenovirus tumorigenic cells. Virology. 2003;306(1):68–76. doi: 10.1016/s0042-6822(02)00079-x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Dahlo M, Isaksson A, Syvanen AC, Pettersson U. The transcriptome of the adenovirus infected cell. Virology. 2012;424(2):115–128. doi: 10.1016/j.virol.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Zhao H, Granberg F, Pettersson U. How adenovirus strives to control cellular gene expression. Virology. 2007;363(2):357–375. doi: 10.1016/j.virol.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Stamminger T, Hearing P. E2F/Rb Family Proteins Mediate Interferon Induced Repression of Adenovirus Immediate Early Transcription to Promote Persistent Viral Infection. PLoS Pathog. 2016;12(1):e1005415. doi: 10.1371/journal.ppat.1005415. [DOI] [PMC free article] [PubMed] [Google Scholar]