Abstract
Context
Pain management is suboptimal in nursing homes.
Objectives
To estimate the extent to which receipt of hospice in nursing homes (NH) increases the receipt of pain management for residents with cancer at the end-of-life.
Methods
Study participants included Medicare beneficiaries with cancer who were NH residents in the last 90 days of life in 2011-2012 (n=78,160). Residents in pain on hospice were matched to like residents without hospice by facility, type of pain assessment (self-report/staff assessment), and weeks until death (9,064 matched strata, 16,968 unique residents. Minimum Data Set 3.0 provided information on residents’ pain prevalence and receipt of pain management (scheduled analgesics, as needed [PRN] medication, non-pharmacologic interventions). We developed conditional logistic models to estimate the association between hospice use and pain management, stratified by self-reported and staff-assessed pain.
Results
We found that pain prevalence was higher in residents using hospice versus those without hospice (e.g. residents who self-reported pain: hospice: 59.9%, 95% Confidence Intervals (CI)=59.3–60.5%; non-hospice: 50.0%, 95% CI=49.4–50.6%). In matched analyses, untreated pain was uncommon (self-reported pain: 2.9% and 5.6% in hospice users and non-users, respectively. Hospice use was associated with receipt of scheduled analgesics (self-reported: adjusted Odds Ratio (aOR) =1.85, 95% CI=1.73–1.971and PRN medication (self-reported: aOR=1.31, 95% CI=1.20–1.43). Pain prevalence and the association between hospice and pain management were similar in residents with staff-assessed pain.
Conclusion
Untreated pain at the end-of-life among residents with cancer in NHs is unusual. Hospice is associated with increased pain management among those with documented pain.
Keywords: Nursing home, hospice, cancer, pain, pain management
INTRODUCTION
In 2009, one of six Medicare decedents with cancer died in a nursing home (NH)1. The majority experienced pain 2, the most common symptom of cancer in older adults3,4. Cancer pain can be effectively treated in most patients using clinical guidelines5–8, and the alleviation of pain is a primary goal of dying residents and their families 9–11. Despite this, the prevalence of untreated and undertreated cancer pain in NHs has been reported to be unacceptably high 12,13.
Barriers to effective pain management in NHs include limited physician visits, inadequate staffing, and an organizational culture that prioritizes improving and maintaining resident function over providing palliative care 14–16. Dying NH residents often only have access to palliative care through enrollment into hospice care17,18, available to Medicare beneficiaries with a life expectancy ≤6 months and who agree to forgo curative treatment19. Hospice providers improve pain management through their expertise in pain assessment and analgesic use. Although NH residents who experience pain and are enrolled in hospice care are more likely to receive analgesics than those receiving traditional care 20,21, there is room for improvement. In NHs, 15% and 25% have untreated pain in long-term and short-term NH hospice, respectively 20,21. These estimates were derived from data sources >15 years old before Centers for Medicare and Medicaid Services (CMS) introduced pain quality indicators (2002), implemented Medicare Part D (2006), strengthened surveyor guidance (F-tag 309;2008), and overhauled pain measures on the Minimum Data Set 3.0 (MDS) in 2010.
Given wide changes in NH policy, we sought to update the estimates of pain prevalence and treatment in hospice-eligible NH residents, and estimate the extent to which hospice enrollment increases pain management in dying NH residents with cancer pain, by leveraging national, comprehensive NH data from 2011 and 2012.
METHODS
Data
We linked vital statistics of Medicare beneficiaries from the Medicare Master Beneficiary Summary File to the MDS 3.0 (2011 and 2012). The MDS 3.0 is a federally required, standardized assessment for all residents living in Medicare- or Medicaid approved NHs (approximately 96% of US NHs). The MDS includes over 450 items on NH residents’ functional status, mood, medical conditions, treatment provided, and other measures22–26. Registered nurses at the NH facility review medical records, observe residents, and communicate with the resident and family members to complete the MDS. Comprehensive assessments are required at admission, annually, and at significant changes in health status including enrollment into hospice. Quarterly assessments (with a subset of items) are conducted every 90 days.
The University of Massachusetts Medical School Review Board approved this study.
Study Sample
We identified all Medicare beneficiaries with a validated date of death in 2011-2012 who had a comprehensive or quarterly MDS assessment ≤90 days before death (n=656,202). MDS assessments completed after a resident’s death date were excluded. Residents with an active MDS diagnosis of cancer listed (item I0100 or an ICD-9 code 140.xx-203.xx listed under I0800) were eligible (n=88,888). We excluded 10,728 decedents who were ≤65 years of age (n=5,311), comatose (n=429), or missing data on key variables (n=4,988). The source population included 78,160 residents. The last MDS assessment before death was used to measure hospice use, pain, pain management, and potential confounders.
Measuring Hospice Use
Hospice is available to all Medicare beneficiaries with a terminal illness and a life expectancy of < 6 months 19. We assumed all in the source population were eligible for hospice. The NH nurse completing the last MDS assessment documented whether NH residents received state licensed and/or Medicare certified hospice care in the previous 14 days (or since admission) while a NH resident or before admission/reentry (MDS 3.0 item O0100K2; Yes/No). If a NH resident received hospice care before NH entry and not during the NH stay, we did not consider them as having received hospice because of potential differences of hospice services in institutionalized settings 27. We did not exclude persons who received hospice care outside of the NH. Receipt of hospice care showed excellent agreement between research nurses and field nurses (κ=0.89)22.
Pain and Pain Management
The outcomes of interest were pain and pain management at the last MDS assessment. All MDS 3.0 assessments collect information on pain presence, frequency, and severity within a five day look back period through either self-report when residents are able to be understood or through staff assessment. For residents who self-reported pain, frequency (rarely, occasionally, frequently, almost constantly) and severity of pain was collected. Severity was measured using either the Verbal Descriptor Scale which categorizes pain as mild, moderate, severe, or very severe/horrible or the Numeric Rating Scale which rates pain from mild to very/severe horrible pain on a scale from 0-10. A validated crosswalk was used to compare the two scales28. We used the CMS quality indicator definition to classify self-reported pain 29. The three categories were no pain, mild/infrequent pain (mild to severe pain occurring rarely/occasionally), and moderate/severe pain (either moderate/severe pain occurring frequently or almost constantly or very severe/horrible pain occurring at any frequency). If NH staff assessed pain, the nurse reviewed the resident’s medical record, consulted other staff, and directly observed the resident to document pain indicators (crying, moaning, grimaces, etc.) and pain frequency in the previous five days (none, occurring 1-2 days, 3-4 days, or daily).We categorized staff-assessed pain as: no pain, infrequent pain (1-2 days), or frequent pain (≥3 days).
Pain management in the 5 days preceding the assessment was based on a medical record review conducted by NH staff of medications and interventions prescribed with the goal of treating pain. Three items were available including receipt of: 1) scheduled pain regiment, 2) any PRN pain medication, and 3) any non-pharmacological pain intervention (including bio-feedback, massage, physical therapy, stretching and strengthening exercises, chiropractic, electrical stimulation, acupuncture, etc.). We categorized receipt of any pharmacologic pain intervention if the assessor recorded a scheduled pain regimen or PRN medication use. Because scheduled pain regiments and PRN medications are differing but complimentary strategies to managing cancer pain30, we examined them separately. All measures of pain and pain management had excellent reliability (κ > 0.92)22.
Potential Confounders
We evaluated resident characteristics that could potentially confound the relationship between receipt of hospice and pain/pain management. Sociodemographic characteristics included age (65-74 years, 75-84 years, ≥85 years), race/ ethnicity (non-Hispanic white vs. other), and marital status (married vs. other). Length of NH stay (<90 days, ≥90 days), type of MDS assessment (admission, quarterly, annual, significant change in status), and whether the resident was dually eligible for Medicaid were considered. Behavioral characteristics included rejection of care in the previous week. Clinical characteristics included comorbidities associated with hospice enrollment (heart failure, dementia) or pain (e.g., hip fracture, diabetes), physical functioning, and cognitive impairment. Physical functioning was measured using the MDS-ADL Self-Performance Hierarchy categorized as totally dependent (5-6) or not (0 to 4).25 Cognitive impairment was measured using the Cognitive Function Scale (CFS),26 which integrates the self-reported Brief Interview for Mental Status (BIMS) with the staff-assessed Cognitive Performance Scale (CPS) when residents could not complete the BIMS screener. We categorized residents by whether they were severely cognitively impaired (incomplete BIMS and a CPS score of (5 or 6) or not (BIMS 0-15 or CPS 0-4).
Matching
Among those with documented pain, we used matching with replacement to group each resident receiving hospice to up to 5 residents not receiving hospice by 1) facility, 2) type of pain assessment received (e.g., whether the resident self-reported pain or had staff observe/assess their pain), and 3) weeks from last MDS assessment to death (+/− 3 days)31. We matched on facility to reduce the potential for facility-level confounding and ascertainment bias in pain assessment32. We matched on type of pain assessment (self-reporting or staff-assessed) because the MDS measures of pain frequency and severity differ. Residents unable to self-report may be more severely cognitively impaired and not comparable to residents who could self-report due to under-ascertainment and under-treatment of pain in this vulnerable subpopulation 24,33,34.
Analysis
All analyses were stratified by self-report versus staff-assessed pain. We conducted analyses to describe resident characteristics and examined distributions of pain reported by hospice use and type of pain assessment in the entire source population. To estimate the association between hospice use and pain management, we used conditional logistic models to account for the matched study design. We included age, race/ethnicity, and gender into all models due to differences in hospice enrollment and receiving analgesics for pain by these factors13,35,36. To evaluate additional confounders, we used a manually driven, step-wise model building process to derive the most parsimonious model that adjusted for confounders. This iterative process required that each potential confounder be included separately into the model to evaluate the effect estimate of interest. The variable that changed the effect estimate the most (providing the change was >10%) was included in the subsequent model; this process continued until the estimate was no longer altered by additional variables. From the full model, we estimated adjusted odds ratios (aOR) and 95% confidence intervals (CI). We conducted an analysis that also stratified by length of NH stay because we hypothesized that residents who are new nursing home residents may be differentially at risk for untreated pain12,13,37. We performed a sensitivity analysis examining only residents in the most pain to address concerns of confounding by pain severity. Because we could not adjust for osteoporosis and arthritis on quarterly MDS assessments, we performed a sensitivity analysis to examine these potential confounders in matched pairs where both residents completed comprehensive MDS assessments.
RESULTS
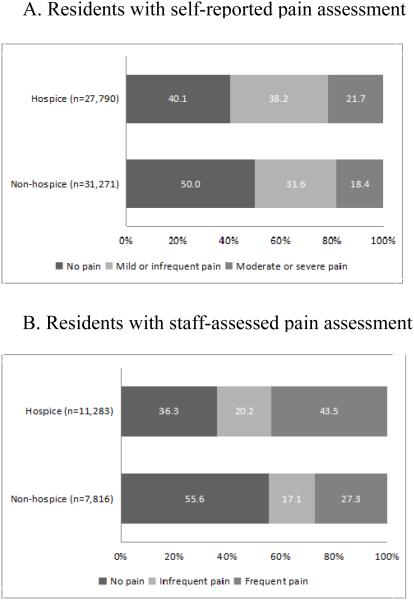
Figure 1 shows the prevalence of pain for NH residents in the source population by type of pain assessment and hospice use. For residents able to self-report pain (Figure 1, Panel A), hospice users were more likely to report any pain than non-hospice users (hospice: 59.9%, 95% CI=59.3–60.5%; non-hospice: 50.0%, 95% CI= 49.4–50.6%). The overall difference was more strongly driven by differences in mild/infrequent pain (hospice: 38.2%, 95% CI=37.6–38.8%; non-hospice: 31.6%, 95% CI= 31.1–32.2%) than moderate to severe pain (hospice: 21.7, 95% CI= 21.2–22.2; non-hospice: 18.4, 95% CI= 17.9–18.8). For residents with staff-assessed pain (Figure 1, Panel B), the overall difference in pain observed (hospice: 63.7% 95% CI= 62.8–64.6; non-hospice: 44.4%, 95% CI= 43.3-45.5%) was primarily driven by reports of frequent pain (hospice: 43.5% 95% CI= 42.6–444.4; non-hospice: 27.3%, 95% CI= 26.3 –28.3%) than infrequent pain (hospice: 20.2% 95% CI= 19.4–20.9; non-hospice: 17.1%, 95% CI= 16.3 – 18.0%). Of those experiencing pain and included in the matched analysis (23,830 hospice users [55.5% of all hospice users] and 19,104 non-hospice users [44.5% of all non-hospice users]), 9,064 hospice users were matched to 7,904 non-hospice users sampled with replacement to create 9,064 matched strata from 1,578 nursing homes.
Figure 1.
Prevalence of pain in source population by hospice use and pain assessment type in nursing home residents with cancer during last 90 days of life (N=78,160)
Table 1 shows characteristics of the matched hospice users and non-hospice users by pain assessment type. Eighteen percent had staff-assessed pain. Median time from the last MDS assessment to death was 3.0 weeks for self-reporting residents (interquartile range [IQR], 1.7 – 5.3) and 0.9 weeks for staff-assessed residents (IQR, 0.3 – 2.1). Matched residents were predominately white regardless of pain assessment type or hospice use. There were differences between residents who self-reported pain and residents with staff-assessed pain. Relative to those who self-reported pain, staff-assessed residents were older and more likely to have a significant change in status MDS assessment, extensive ADL compromise, severe cognitive impairment, and dementia. Hospice users had similar levels of severe cognitive impairment in comparison to non-hospice users (regardless of pain assessment type), but were more likely to have a significant change in status MDS assessment and be totally physically dependent. Hospice users (regardless of assessment type) were more likely to have anxiety disorders relative to non-users but had similar prevalence of other comorbid conditions with the exception of surgical wounds in residents who self-reported pain only. In those who could self-report pain only, hospice users were more likely to have dual eligibility for Medicare/Medicaid and were less likely to have an admission/quarterly/annual MDS assessment or be married relative to non-users. In those with staff-assessed pain only, hospice users were less likely to be long-term nursing home residents and have a quarterly or annual MDS assessment relative to non-users.
Table 1.
Characteristics of matched nursing home residents with cancer experiencing any pain by assessment type and hospice exposure (N=16,968)a
| Characteristicb | Self-report | Staff assessment | ||
|---|---|---|---|---|
|
|
|
|||
| Hospice, n=7,242 |
Non-hospice, n=6,694 |
Hospice, n=1,822 |
Non-hospice, n= 1,210 |
|
| Weeks to death, median (IQR) c | 2.9 (1.6-5.0) | 3.3 (1.9-5.6) | 0.9 (0.3-1.9) | 1.1 (0.4-2.4) |
| Age, years | ||||
| 65-74 | 26.1 | 25.9 | 19.1 | 17.2 |
| 75-84 | 38.4 | 38.5 | 35.4 | 33.3 |
| 85+ | 35.5 | 35.6 | 45.5 | 49.5 |
| Women | 57.9 | 53.2 | 55.6 | 53.1 |
| Marriedd | 25.6 | 31.9 | 33.6 | 34.4 |
| Non-Hispanic white | 88.0 | 86.5 | 86.2 | 87.7 |
| Long-term NH staye | 23.4 | 24.7 | 22.4 | 31.2 |
| Medicaid/Medicare dual eligibility | 48.3 | 40.9 | 44.8 | 46.9 |
| Type of MDS assessment | ||||
| Admission | 43.6 | 58.5 | 42.7 | 43.6 |
| Quarterly | 10.9 | 19.6 | 7.2 | 17.1 |
| Annual | 1.2 | 4.1 | 0.7 | 3.6 |
| Significant Change in Status | 44.3 | 17.7 | 49.3 | 35.7 |
| Extensive ADL compromisef | 47.8 | 35.6 | 75.3 | 62.5 |
| Severe cognitive impairmentg | 4.3 | 2.4 | 41.3 | 38.9 |
| Reject care | 10.8 | 9.9 | 18.6 | 18.5 |
| Comorbidities conditions that could be primary indications for hospiceh |
||||
| Heart failure | 19.4 | 23.3 | 17.5 | 19.9 |
| Dementia | 20.7 | 19.5 | 41.3 | 45.0 |
| Comorbidities associated with painh | ||||
| Arthritisi | 23.0 | 24.6 | 24.1 | 27.5 |
| Osteoporosisi | 10.8 | 10.5 | 9.9 | 12.9 |
| Hip fracture | 2.4 | 3.6 | 4.0 | 5.0 |
| Other fracture | 4.5 | 6.6 | 3.7 | 5.2 |
| Diabetes mellitus | 26.4 | 32.8 | 24.5 | 27.0 |
| Parkinson’s disease | 3.0 | 3.1 | 4.6 | 5.0 |
| Anxiety disorder | 33.7 | 23.7 | 31.1 | 26.8 |
| Depression | 39.7 | 35.9 | 36.3 | 35.7 |
| Pressure ulcers | 24.5 | 24.7 | 29.9 | 29.6 |
| Surgical wounds | 5.2 | 11.7 | 5.4 | 6.2 |
IQR= Interquartile range, NH= nursing home
Characteristics are displayed for individual nursing home residents and do not account for repeated sampling of non-hospice users. The 16,968 unique residents are grouped into 9,064 matched strata of 9,064 hospice users and 15,875 non-hospice users (7,904 unique non-hospice users are repeatedly sampled on average of 2 times each).
Characteristics are presented as percentages unless otherwise noted.
Represents the weeks from the last resident Minimum Data Set assessment to death.
Missing data on marital status for 119 hospice and 100 non-hospice users who self-reported pain and 26 hospice and 14 non-hospice users who had staff-assessed pain.
Long-term nursing home stay defined as ≥90 days.
Based on four-level scale: Cognitive Function Scale score of 4.
Based on seven-level scale: MDS-ADL Self-Performance Hierarchy score of 5 or 6.
All comorbidities are active diagnoses in previous 7 days with the exception of hip fracture and other fracture, which have a 6 month look back window.
Osteoporosis and arthritis were not measured on quarterly assessments. Percentages represent NH decedents who had comprehensive assessments only including 6,450 hospice and 5,381 non-hospice users who self-reported pain and 1,690 hospice and 1,003 non-hospice users who had staff-assessed pain.
Receipt of Pain Management
Table 2 shows the association of hospice use and receipt of pharmacologic and non-pharmacologic pain management among residents with any pain documented by type of pain assessment. Overall, those receiving hospice had greater odds of receiving medication relative to those who did not receive hospice (aOR (self-reported) = 2.03, 95% CI =1.72–2.39; aOR(staff-assessed)=3.30, 95% CI=2.14–5.09). Relative to those not receiving hospice, those who did were more likely to receive scheduled analgesics (aOR (self-reported) = 1.85, 95% CI=1.73–1.97 1.60, 95% CI=1.48–1.73; aOR (staff-assessed) = 1.45, 95% CI=1.28–1.65) and PRN medication (aOR(self-reported)= 1.31, 95% CI=1.20–1.43, aOR(staff-assessed)= 1.66, 95% CI=1.36–2.04). Non-pharmacologic pain management was less commonly used than medication Hospice users were more likely to receive non-pharmacologic pain management relative to non-hospice users (aOR (self-reported) = 1.18, 95% CI=1.11–1.26; aOR (staff-assessed) = 1.41, 95% CI=1.23–1.61).
Table 2.
Association between hospice use and pain management in nursing home decedents with cancer who experienced any pain (9,064 matched strata)
| Outcome | Hospice, % | Non-hospice, % | Crude OR (95% CI) | Adjusteda OR (95% CI) |
|---|---|---|---|---|
| Self-reported pain assessment (7,242 matched strata)b | ||||
|
| ||||
| Any pharmacologic pain management | 97.1 | 94.4 | 1.99 (1.69–2.34) | 2.03 (1.72–2.39) |
| Scheduled pain regimen | 71.5 | 56.1 | 1.85 (1.74–1.97) | 1.85 (1.73–1.97) |
| PRN medication | 86.9 | 83.1 | 1.32 (1.21–1.44) | 1.31 (1.20–1.43) |
| Non-pharmacologic pain management | 45.1 | 41.1 | 1.19 (1.12–1.27) | 1.18 (1.11–1.26) |
|
| ||||
| Staff pain assessment (1,822 matched strata)c | ||||
|
| ||||
| Any Pharmacologic Pain Management | 98.4 | 95.2 | 3.24 (2.14–4.93) | 3.30 (2.14–5.09) |
| Scheduled Pain Regimen | 68.2 | 58.2 | 1.48 (1.30–1.68) | 1.45 (1.28–1.65) |
| PRN Medication | 90.7 | 84.1 | 1.67 (1.37–2.04) | 1.66 (1.36–2.04) |
| Non-pharmacologic pain management | 50.8 | 40.3 | 1.41 (1.23–1.61) | 1.41 (1.23–1.61) |
OR = odds ratio, CI = confidence interval
Adjusted for age, gender, and race/ethnicity
7,242 hospice users matched to 13,387 non-hospice users (6,694 unique non-hospice users matched with replacement).
1,822 hospice users matched to 2,488 non-hospice users (1,210 unique non-hospice users matched with replacement).
Table 3 shows the analysis stratified by length of NH stay. For residents who self-reported pain, hospice use was associated with receipt of scheduled analgesics in short-term residents (aOR (self-report) =1.90, 95% CI=1.75–2.05) and long-term residents (aOR (self-report) =1.87, 95% CI=1.46–2.39). Hospice use was associated with scheduled analgesics in residents with staff-assessed pain for short-term residents only (aOR(staff-assessed)= 1.45, 95% CI=1.23–1.70).
Table 3.
Association between hospice use and pain management in nursing by length of stay in nursing home residents with cancer who experienced any pain (6,696 matched strata)a
| Short-term resident (< 90 days) | Long-term resident (≥90 days) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Outcome | Hospice, % | Non-hospice, % | Adjusteda OR (95% CI) |
Hospice, % | Non-hospice, % | Adjustedb OR (95% CI) |
| Self-reported pain assessment (4,752 short-term matched strata, 703 long-term matched strata)c | ||||||
|
| ||||||
| Any pharmacologic pain management | 97.4 | 94.6 | 2.02 (1.63–2.51) | 96.2 | 94.5 | 1.25 (0.75–2.09) |
| Scheduled pain regimen | 68.9 | 52.4 | 1.90 (1.75–2.05) | 80.4 | 69.3 | 1.87 (1.46–2.39) |
| PRN medication | 89.4 | 87.2 | 1.18 (1.05–1.33) | 79.4 | 71.0 | 1.47 (1.14–1.89) |
| Non-pharmacologic pain management | 45.6 | 42.7 | 1.14 (1.05–1.24) | 41.7 | 35.8 | 1.34 (1.07–1.68) |
|
| ||||||
| Staff pain assessment (1,072 short-term matched strata, 169 long-term matched strata)d | ||||||
|
| ||||||
| Any pharmacologic pain management | 98.0 | 95.6 | 2.63 (1.51–4.57) | 97.6 | 94.2 | 3.83 (0.83–17.57) |
| Scheduled pain regimen | 64.8 | 53.8 | 1.45 (1.23–1.70) | 76.9 | 71.4 | 1.21 (0.73–2.02) |
| PRN medication | 92.8 | 89.7 | 1.62 (1.20–2.20) | 83.4 | 69.5 | 2.19 (1.25–3.84) |
| Non-pharmacologic pain management | 52.4 | 41.2 | 1.43 (1.19–1.71) | 44.4 | 35.1 | 1.61 (0.99–2.63) |
OR = odds ratio, CI = confidence interval
Of the total 9,064 matched strata, 2,368 matched strata were not included in this analysis because of complete discordance of length of stay between hospice users and non-hospice users.
Adjusted for age, gender, and race/ethnicity
In sensitivity analyses including only NH residents who self-reported severe pain or who had staff-assessed frequent pain, the association between hospice use and pain management remained for receiving any medication (aOR(self-reported)=2.62, 95% CI= 1.76–3.91; aOR(staff-assessed)=4.41, 95% CI= 2.03–9.58), scheduled analgesics(aOR(self-reported)=1.71, 95% CI=1.47–1.99; aOR(staff-assessed)=1.46, 95% CI=1.21–1.75), PRN medication (aOR(self-reported)=1.61, 95% CI=1.30–2.00; aOR(staff-assessed)=1.48, 95% CI=1.09–2.01), and nonpharmacologic pain management (aOR(self-reported)=1.24, 95% CI=1.07–1.45; aOR(staff-assessed)=1.48, 95% CI=1.09–2.01).
Analyses using only full MDS assessments showed no evidence that the effect estimates were confounded by arthritis or osteoporosis (data not shown).
DISCUSSION
To our knowledge, this is one of the first national and comprehensive studies to focus on hospice care and pain/pain management in NH residents with cancer at the end-of-life. We found that among residents who self-reported pain, hospice use was associated with receipt of analgesics including both scheduled and PRN medication in comparison to non-hospice residents. Untreated pain in hospice and non-hospice users was lower than previous estimates20,38, but pain management strategies varied by hospice use and assessment type. The prevalence of pain in our source population was higher in NH residents receiving hospice care compared to residents not receiving hospice care.
The largest previous study of hospice and pain management in NHs using data from 1992 to 1996 found that untreated pain was 15% in hospice users and 23% in non-hospice users20. Similarly, a study using national survey data from 2004 found that approximately 15% of hospice users had untreated pain38. Our findings suggest that there was an increase in overall use of pharmacologic pain management in both hospice and non-hospice users. We are uncertain what may have led to this increase in overall use, but our findings suggests that the largest change is in PRN medication use, as our estimates for scheduled analgesics use are consistent with estimates of use in NH residents using hospice from 200438. Although it is possible that this increase could be due to limiting our sample to residents with cancer, we find this unlikely due to the similar poor quality of pain management in NHs regardless of whether residents have a cancer diagnosis39,40.
Current guidelines recommend a multimodal/proactive approach to cancer pain that includes scheduled and PRN pain medication, accompanied by non-pharmacologic interventions30. PRN medication use was fairly high regardless of hospice use or pain assessment type, but there was wide variation in scheduled analgesics use. Residents receiving hospice care had increased odds of receiving scheduled analgesics relative to non-hospice residents, but overall use of scheduled analgesics ranged from 52-80%. Further, we found that lack of scheduled analgesic use was common among those in the most pain, indicating that pain severity was no guarantee of receiving guideline recommended pain medication. Although it appears that increases in pain management have occurred in NHs, improvements are still possible. The data available in the current study do not permit comment on the quality of analgesic medications used.
We found that hospice use was associated with non-pharmacologic interventions. These interventions were used less frequently than medications, and it is unclear if hospice users received different interventions than non-hospice users. The MDS does not capture details regarding the specific types of non-pharmacological interventions used. Common non-pharmacologic interventions for cancer pain include hot/cold therapy, physical therapy, nerve blocks, electrical stimulation, bio-feedback and acupuncture41,42. Non-pharmacologic interventions can be useful and effective as primary adjuvants in the treatment of cancer pain, though how they are used in NHs is unclear.
The association between hospice and receipt of scheduled analgesics was strongest in short-term residents. This appears to be driven less by the high level of scheduled analgesics in hospice users and more by the relatively low use of scheduled analgesics in short-term residents not using hospice. Non-hospice users receive both their custodial care and pain management from NH staff who may not be adequately trained to provide appropriate pain management and may not know the resident when they initially transition to the NH 37, making it difficult to appropriately treat pain. Hospice care may be particularly useful during this initial transition because hospice staff specialize in pain and symptom management and have experience treating patients that they have never seen before. The relative benefits of hospice care are largely present in long-term NH residents as well, though the overall use of scheduled analgesics increased regardless of hospice status.
In the source population, pain was reported in more than half of all NH residents with cancer at the end-of-life, and hospice users reported more pain than non-hospice users. Some of the differences in pain by hospice use may be due to how and when NH residents receive hospice care, as NH staff members have cited unmanageable pain as a common reason for referring residents to hospice43. However, there is potential for ascertainment bias if residents receiving hospice were more likely to have their pain recorded32,44, and this may be differential by pain assessment type. Residents who could self-report likely received a standardized pain interview from facility nurses administering the MDS assessment, but when pain was staff-assessed, hospice users may have received enhanced pain surveillance and that resulted in more pain recorded relative to non-users. In this case, the frequency of pain and untreated pain may be under-documented in non-hospice users with staff-assessed pain; this could have biased our findings towards the null for this subgroup.
This study has several strengths. It provides a needed update on pain management in hospice and non-hospice residents at the end-of-life that is specific to cancer; it includes a comprehensive, recent national sample of Medicare beneficiaries in NHs; and uses enhanced MDS 3.0 assessments with improved pain interviews. This study also has limitations. First, hospice users may have stronger desires for pain management than non-hospice users. However, in our sensitivity analyses, we found that the positive association between hospice and receiving analgesics was still present in those reporting the most pain (and hypothesized to most desire pain management) . Further, prior studies document that dying residents and their families desire effective pain management regardless of whether the resident is enrolled in hospice 10,11. Second, we could not evaluate specific pharmacologic and non-pharmacologic interventions received, we could only comment on the use of general pain management strategies recorded in the MDS. Third, we lacked information on cancer type and severity, which may have implications for whether the pain experienced was due to cancer. However, pain management guidelines are not cancer specific and the outcomes we considered measured what should be minimally done for residents experiencing pain at the end-of-life. Fourth, we had limited information on hospice use and could not evaluate the potentially moderating effects of length of hospice stay.21 Finally, this study only considered physical pain; psychological, existential, and spiritual pain were not considered.
CONCLUSION
The study provides an important update on pain management in NH residents at the end-of-life. Given that pain management is one of the primary goals of dying residents and their families, our finding that hospice use was associated with receiving pain management in residents with any reported or observed pain suggests that hospice care may contribute to a better quality of life at the end-of-life. We also found that untreated pain was lower than previous estimates for both hospice and non-hospice users, which may suggest that the overall quality of life for dying residents with cancer is improving. However, future work is needed to examine appropriateness of medications used, as the overall prevalence of pain was extensive.
ACKNOWLEDGEMENTS
This work was supported by the National Cancer Institute [grant 1R21CA198172] and the National Center for Advancing Translational Sciences [1TL1TR001454].
Funding Sources and related paper presentations: This work was funded by grants to Jacob Hunnicutt and Kate Lapane (1R21CA198172 and 1TL1TR001454).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interests.
Author Contributions:
JNH was responsible for the study concept and design, analysis and interpretation of data, and preparation of the manuscript.
JT was responsible for the study concept and design, interpretation of data, and critical revision of the manuscript.
KLL was responsible for the study concept and design, interpretation of data, and critical revision of the manuscript.
Sponsor’s Role: None
REFERENCES
- 1.Teno JM, Gozalo PL, Bynum JPW, et al. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309:470–477. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drageset J, Corbett A, Selbaek G, et al. Cancer-related pain and symptoms among nursing home residents: a systematic review. J Pain Symptom Manage. 2014;48:699–710. doi: 10.1016/j.jpainsymman.2013.12.238. e1. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson NA, Given CW. Determinants of functional recovery in older adults surgically treated for cancer. Cancer Nurs. 2004;27:10–16. doi: 10.1097/00002820-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rao A, Cohen HJ. Symptom management in the elderly cancer patient: fatigue, pain, and depression. J Natl Cancer Inst Monogr. 2004;32:150–157. doi: 10.1093/jncimonographs/lgh031. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization . Cancer pain relief. World Health Organization; Geneva, Switzerland: 1996. [Google Scholar]
- 6.American Society of Anesthesiologists Task Force on Pain Management, Cancer Pain Section Practice guidelines for cancer pain management. A report by the American Society of. Anesthesiologists Task Force on Pain Manaement, Cancer Pain Section. Anesthesiology. 1996;84:1243–1257. [PubMed] [Google Scholar]
- 7.American Geriatrics Society Panel on Pharmacological management of Persistent Pain in Older Persons. J Am Geriatr Soc. 2009;57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 8.Ripamonti CI, Santini D, Maranzano E, et al. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl 7):139–154. doi: 10.1093/annonc/mds233. [DOI] [PubMed] [Google Scholar]
- 9.Bernabei R, Gambassi G, Lapane K, et al. Management of pain in elderly patients with cancer. SAGE Study Group. Systematic Assessment of Geriatric Drug Use via Epidemiology. JAMA. 1998;279:1877–1882. doi: 10.1001/jama.279.23.1877. [DOI] [PubMed] [Google Scholar]
- 10.Pimentel CB, Briesacher BA, Gurwitz JH, et al. Pain Management in Nursing Home Residents with Cancer. J Am Geriatr Soc. 2015;63:633–641. doi: 10.1111/jgs.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Medicine . Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Institute of Medicine; Washington, DC: 2015. [DOI] [PubMed] [Google Scholar]
- 12.Steinhauser KE, Christakis NA, Clipp EC, et al. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284:2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- 13.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;29:88–93. doi: 10.1001/jama.291.1.88. [DOI] [PubMed] [Google Scholar]
- 14.Oliver DP, Porock D, Zweig S. End-of-life care in U.S. nursing homes: a review of the evidence. J Am Med Dir Assoc. 2004;5:147–155. doi: 10.1097/01.JAM.0000123063.79715.8E. [DOI] [PubMed] [Google Scholar]
- 15.Shield RR, Wetle T, Teno J, et al. Physicians “missing in action”: family perspectives on physician and staffing problems in end-of-life care in the nursing home. J Am Geriatr Soc. 2005;53:1651–1657. doi: 10.1111/j.1532-5415.2005.53505.x. [DOI] [PubMed] [Google Scholar]
- 16.Kayser-Jones J, Schell E, Lyons W, et al. Factors that influence end-of-life care in nursing homes: the physical environment, inadequate staffing, and lack of supervision. Gerontologist. 2003;43:76–84. doi: 10.1093/geront/43.suppl_2.76. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson DG, Bramson JS. Hospice care in the nursing home setting: a review of the literature. J Pain Symptom Manage. 2009;38:440–451. doi: 10.1016/j.jpainsymman.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Miller SC, Lima J, Gozalo PL, et al. The growth of hospice care in US nursing homes. J Am Geriatr Soc. 2010;58:1481–1488. doi: 10.1111/j.1532-5415.2010.02968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CMS Manual System Pub 100-02 Medicare Benefit Policy Manual. Chapter 9 - Coverage of hospice services under hospital insurance. (Rev 209; Issued 05-08-15). Available from: https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/bp102c09.pdf. Accessed August 15, 2016.
- 20.Miller SC, Mor V, Wu N, et al. Does receipt of hospice care in nursing homes improve the management of pain at the end of life? J Am Geriatr Soc. 2002;50:507–515. doi: 10.1046/j.1532-5415.2002.50118.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller SC, Mor V, Teno J. Hospice enrollment and pain assessment and management in nursing homes. J Pain Symptom Manage. 2003;26:791–799. doi: 10.1016/s0885-3924(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 22.Saliba D, Buchanan J. Development and validation of a revised nursing home assessment tool: MDS 3.0. Rand Corp. Santa Monica; 2008. [Google Scholar]
- 23.Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc. 2012;13:602–610. doi: 10.1016/j.jamda.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Saliba D, Jones M, Streim J, et al. Overview of significant changes in the Minimum Data Set for nursing homes version 3.0. J Am Med Dir Assoc. 2012;13:595–601. doi: 10.1016/j.jamda.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54:546–553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 26.Thomas KS, Dosa D, Wysocki A, et al. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care. 2015 doi: 10.1097/MLR.0000000000000334. Available from: http://journals.lww.com/lww-medicalcare/Abstract/publishahead/The_Minimum_Data_Set_3_0_Cognitive_Function_S cale_.99066.aspx. Accessed August 15, 2016. [DOI] [PMC free article] [PubMed]
- 27.Stevenson DG, Huskamp HA, Grabowski DC, et al. Differences in hospice care between home and institutional settings. J Palliat Med. 2007;10:1040–1047. doi: 10.1089/jpm.2007.0071. [DOI] [PubMed] [Google Scholar]
- 28.Edelen MO, Saliba D. Correspondence of verbal descriptor and numeric rating scales for pain intensity: an item response theory calibration. J Gerontol A Biol Sci Med Sci. 2010;65:778–785. doi: 10.1093/gerona/glp215. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Medicare and Medicaid Services Nursing Home Data Compendium. 2013 Available from: https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/downloads/nursinghomedatacompendium_508.pdf. Accessed August 15, 2016.
- 30.Gordon DB, Dahl JL, Miaskowski C, et al. American Pain Society recommendations for improving the quality of acute and cancer pain management: American Pain Society Quality of Care Task Force. Arch Intern Med. 2005;165:1574–1580. doi: 10.1001/archinte.165.14.1574. [DOI] [PubMed] [Google Scholar]
- 31.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu N, Miller SC, Lapane K, et al. The quality of the quality indicator of pain derived from the minimum data set. Health Serv Res. 2005;40:1197–1216. doi: 10.1111/j.1475-6773.2005.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen-Mansfield J, Lipson S. Pain in cognitively impaired nursing home residents: how well are physicians diagnosing it? J Am Geriatr Soc. 2002;50:1039–1044. doi: 10.1046/j.1532-5415.2002.50258.x. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds KS, Hanson LC, DeVellis RF, et al. Disparities in pain management between cognitively intact and cognitively impaired nursing home residents. J Pain Symptom Manage. 2008;35:388–396. doi: 10.1016/j.jpainsymman.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Keating NL, Herrinton LJ, Zaslavsky AM, et al. Variations in hospice use among cancer patients. J Natl Cancer Inst. 2006;98:1053–1059. doi: 10.1093/jnci/djj298. [DOI] [PubMed] [Google Scholar]
- 36.Cohen LL. Racial/ethnic disparities in hospice care: a systematic review. J Palliat Med. 2008;11:763–768. doi: 10.1089/jpm.2007.0216. [DOI] [PubMed] [Google Scholar]
- 37.Rodin MB. Cancer patients admitted to nursing homes: what do we know? J Am Med Dir Assoc. 2008;9:149–156. doi: 10.1016/j.jamda.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Hanlon JT, Perera S, Sevick MA, et al. Pain and its treatment in older nursing home hospice/palliative care residents. J Am Med Dir Assoc. 2010;11:579–583. doi: 10.1016/j.jamda.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapane KL, Quilliam BJ, Chow W, et al. Pharmacologic management of non-cancer pain among nursing home residents. J Pain Symptom Manage. 2013;45:33–42. doi: 10.1016/j.jpainsymman.2011.12.285. [DOI] [PubMed] [Google Scholar]
- 40.Won AB, Lapane KL, Vallow S, et al. Persistent nonmalignant pain and analgesic prescribing patterns in elderly nursing home residents. J Am Geriatr Soc. 2004;52:867–74. doi: 10.1111/j.1532-5415.2004.52251.x. [DOI] [PubMed] [Google Scholar]
- 41.Portenoy RK. Treatment of cancer pain. Lancet. 2011;377:2236–2247. doi: 10.1016/S0140-6736(11)60236-5. [DOI] [PubMed] [Google Scholar]
- 42.Pan CX, Morrison RS, Ness J, et al. Complementary and alternative medicine in the management of pain, dyspnea, and nausea and vomiting near the end of life. A systematic review. J Pain Symptom Manage. 2000;20:374–387. doi: 10.1016/s0885-3924(00)00190-1. [DOI] [PubMed] [Google Scholar]
- 43.Welch LC, Miller SC, Martin EW, et al. Referral and timing of referral to hospice care in nursing homes: the significant role of staff members. Gerontologist. 2008;48:477–484. doi: 10.1093/geront/48.4.477. [DOI] [PubMed] [Google Scholar]
- 44.Wu N, Miller SC, Lapane K, et al. The problem of assessment bias when measuring the hospice effect on nursing home residents’ pain. J Pain Symptom Manage. 2003;26:998–1009. doi: 10.1016/s0885-3924(03)00328-2. [DOI] [PubMed] [Google Scholar]