Abstract
Objectives
To compare the risk of serious adverse events, serious infections and death caused by methotrexate and biological disease-modifying antirheumatic drug (bDMARD) combination therapy versus a bDMARD prescribed as monotherapy in rheumatoid arthritis (RA).
Methods
A systematic literature review was conducted until February 2016 in PubMed, Embase and Cochrane Library databases by selecting randomised controlled trials comparing methotrexate and bDMARD combination therapy to bDMARD monotherapy in RA. The meta-analysis compared the occurrence of (1) serious adverse events, (2) serious infections and (3) death among these groups by the Mantel-Haenszel method.
Results
The literature review selected 16 controlled trials comparing methotrexate and bDMARD combination therapy to bDMARD monotherapy. After meta-analysis comparing patients under monotherapy to those under combination therapy: (1) the risk of occurrence of serious adverse events was comparable in 12 trials: RR (95% CI) 0.92 (0.78 to 1.08). (2) No significant difference was observed in the risk of occurrence of serious infections in 13 trials: RR (95% CI) 1.15 (0.84 to 1.58). We noted a trend, although insignificant, towards a high risk of the occurrence of tuberculosis in 10 studies: RR (95% CI) 1.78 (0.63 to 4.99). (3) The risk of death was comparable in 12 trials: RR (95% CI) 0.73 (0.40 to 1.35).
Conclusions
The results showed no significant difference between the two groups, confirming that the use of methotrexate and bDMARD combination therapy in RA does not cause an increased risk of serious adverse events or serious infections or death compared with bDMARD monotherapy.
Keywords: Rheumatoid Arthritis, DMARDs (biologic), Methotrexate
Key messages.
What is already known about this subject?
Addition of methotrexate to a biological disease-modifying antirheumatic drug (bDMARD) does not increase risk of severe adverse events in rheumatoid arthritis (RA).
What does this study add?
Addition of methotrexate to a bDMARD does not increase risk of serious infections in RA.
How might this impact in clinical practice?
Methotrexate should be prescribed as soon as it is tolerated in addition to bDMARDs.
A non-significant trend towards an increased tuberculosis risk with methotrexate was observed.
According to the EULAR (EUropean League Against Rheumatism) recommendations of 2013,1 a disease-modifying drug should be started as soon as rheumatoid arthritis (RA) is diagnosed with the objective of obtaining remission or at least a low level of activity. Methotrexate is the first-line treatment for use as monotherapy or readily combined with other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs).1 2 When remission is not reached and if RA has poor prognostic factors, a biological disease-modifying antirheumatic drug (bDMARD) is added to methotrexate. Methotrexate and bDMARD combination therapy is more effective than a single bDMARD in most cases with better therapeutic support.1 3 The impact of disease-modifying combination therapy on the risk of side effects compared with bDMARD monotherapy has never been clearly assessed.
The serious infection risk is 2.5 higher in RA compared to the risk in the general population due to immune system disturbances, the use of corticosteroids and disease-modifying therapies.4–7
There are discrepancies in the literature regarding the risk of infection due to methotrexate given as monotherapy in RA.4 8–10 A recent meta-analysis by Singh et al11 showed that, in RA, using the standard dose of bDMARDs (used alone or in combination with csDMARDs) was associated with an increased risk of serious infections compared with csDMARDs alone. Moreover, Lorenzetti et al12 showed that the risk of tuberculosis under anti-TNF-α therapy was nearly 13 times higher when combined with methotrexate compared with its use in monotherapy.
Work by Ruderman13 summarising the main national registers of RA showed that the use of methotrexate was associated with a risk of hepatotoxicity, cytopenias and pneumonia, especially during the first year of administration, as anti-TNF-α is associated with an increased risk of infection (including serious), whether bacterial, fungal or opportunistic.
The aim of our study was to compare the risk of occurrence of serious adverse events, serious infections and death among patients receiving a methotrexate and bDMARD combination compared with those receiving bDMARD monotherapy in RA by a systematic literature review and meta-analysis.
Methods
We undertook this literature review and meta-analysis in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.14
Search strategy
The search strategy consisted of a systematic review of the literature for randomised controlled trials comparing bDMARD and methotrexate combination therapy to bDMARD prescribed as monotherapy in adult patients with RA.
We performed a search in January 2015 in the PubMed, Embase and Cochrane CENTRAL databases to collect all randomised controlled trials since inception. We updated the data in February 2016 for the PubMed and Cochrane Library databases.
The search terms used in PubMed were (‘Arthritis, Rheumatoid’(Mesh)) AND ‘Randomized Controlled Trial’ (Publication Type) AND (ETANERCEPT(TEXT) OR INFLIXIMAB(TEXT) OR ADALIMUMAB(TEXT) OR CERTOLIZUMAB(TEXT) OR GOLIMUMAB(TEXT) OR TOCILIZUMAB(TEXT) OR RITUXIMAB(TEXT) OR ABATACEPT(TEXT) OR ANAKINRA(TEXT)).
We searched the Embase database for articles using the following keywords: (TITLE–ABS–KEY (etanercept OR infliximab OR adalimumab OR certolizumab OR golimumab OR tocilizumab OR rituximab OR abatacept OR anakinra)) AND (TITLE–ABS KEY (arthritis rheumatoid)) AND (LIMIT-TO (SUBJAREA, ‘PHAR’)).
We conducted nine separate searches in the Cochrane Library with the following keywords: bDMARD name+‘RHEUMATOID ARTHRITIS’ AND ‘monotherapy’, giving a total of nine combinations.
We also conducted a search for abstracts of the SFR (French Society of Rheumatology), ACR (American College of Rheumatology) and EULAR annual meetings over the past 3 years (2013, 2014 and 2015).
Article inclusion and exclusion criteria
We selected all randomised controlled trials which compared methotrexate and bDMARD combination therapy to bDMARD monotherapy in adult patients with RA. The bDMARDs included anti-TNF-α (adalimumab, certolizumab, etanercept, golimumab, infliximab), tocilizumab, abatacept, rituximab and anakinra. Patients were either csDMARD-naive or bDMARD-naive or not.
We excluded articles that were not written in English or French, non-randomised trials, studies that did not include two separate randomised groups of methotrexate and bDMARD combination therapy and bDMARDs prescribed as monotherapy.
The selection of articles on reading the title and abstract was carried out by one reader (CB).
Data collection
Data collection for each article was made using a predefined reading grid. This grid was previously tested on three articles to verify the feasibility of the collection. The selection of trials for complete reading and data collection of the articles was performed independently by two individuals (AR-W and CB). Disputes were resolved by consensus.
The following data were collected systematically: study characteristics (article title, journal and publication date, number of patients included, study duration, bDMARD studied, mean methotrexate dose prescribed), population characteristics studied: mean age, sex ratio, rheumatoid factor (RF) status, RA disease duration, number of previous biotherapies administered, corticosteroids (percentage of patients treated with oral corticosteroids during the study and mean daily dose in prednisone equivalent), end points expressed (conversions made if necessary) as the number of events per 100 patient-years (100 PY): serious adverse events, serious infections (with specific collection of tuberculosis cases), death.
Adverse events and infections were considered serious if they met the definition of the FDA (Food and Drug Administration)18 or were considered serious according to the authors of the article.18 In the case of missing data, the authors were contacted by email to retrieve the number of events.
Statistical analysis
We first described the studies included in the meta-analysis and the population of interest. The results are presented according to the nature of the event and divided into three parts: serious adverse events, serious infections, and tuberculosis and death.
We found (or converted into the number of patient-years if necessary) the number of each of these events for each study included in our analysis. For each event, the risk was calculated by meta-analysis by the Mantel-Haenszel fixed effects method. This method provides a fixed number and combines studies using a similar method to the inverse variance approaches to determine the weight given to each study. It provides a common estimate of the risk ratios (RR) and CIs (95% CI). ‘Forest plot’ graphs showing the RR and 95% CI were constructed for each of the evaluation criteria. Statistical heterogeneity of the samples was evaluated using a Q test using a significance level of 0.05 and reported with I2 statistics that describe inconsistency across studies. I2 was interpreted as follows: 0–40%: not important heterogeneity, 30–60%: moderate heterogeneity, 50–90%: substantial heterogeneity, 75–100%: considerable heterogeneity. All analyses were performed using the Revman 5.3 software (Nordic Cochrane Center).
Results
Article selection process
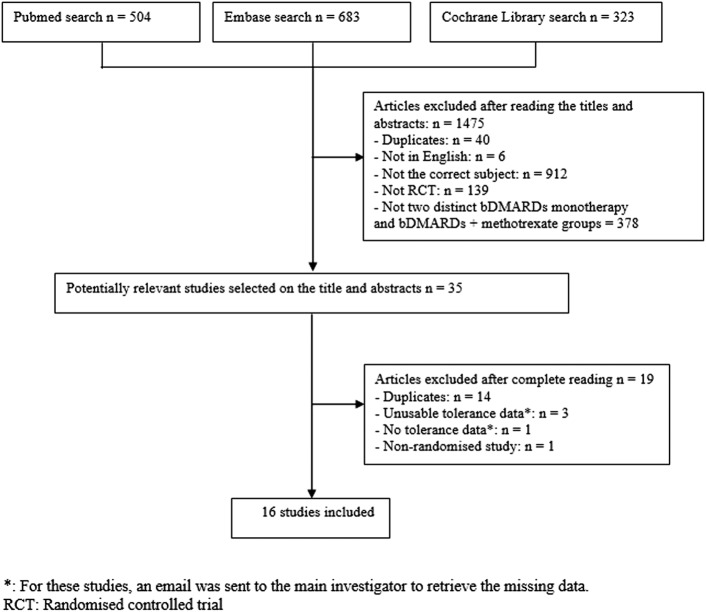
We obtained a total of 1510 articles in the search: 504 articles via PubMed, 683 via Embase and 323 via the Cochrane Library. We excluded 1475 articles after reading the titles and abstracts (see flow chart figure 1) and thus retained 35 articles. After full-length article reading, 19 were eliminated as 14 were duplicates (intermediate results of the same studies), 3 studies expressed unusable tolerance data (unfeasible patient-year conversions) and 1 study provided no safety data and 1 was not a randomised trial. We contacted the authors of the four studies that did not provide enough safety data to be analysed by email, but have not been able so far to retrieve the data needed for the study. Finally, we retained 16 articles.15–30
Figure 1.
Article selection flow chart.
All of these trials included 4975 patients, representing a total of 6764 patient-years followed up and including 3866 patient-years in the ‘bDMARD and methotrexate’ group and 2898 patient-years in the ‘bDMARD alone’ group.
Description of the selected studies
Of these 16 articles, 2 concerned abatacept,18 27 1 concerned adalimumab,15 5 concerned etanercept,17 21 22 29 30 3 concerned golimumab,19 24 28 1 concerned infliximab,25 1 concerned rituximab16 and 3 concerned tocilizumab.20 23 26
Trial quality was assessed using the Jadad scale: 13 articles had a Jadad score ≥3/5 and 3 had a score of 2/5 (see online supplementary table S1 in appendix 1).
The main data concerning the studies are shown in table 1.
Table 1.
Characteristics of the studies included in the meta-analysis
| Studyreference | Publication year | Jadad score (/5) | Number of patients | Follow-up duration (years) | bDMARD | Mean MTX dose (mg/week) |
|---|---|---|---|---|---|---|
| 1. ACCOMPANY27 | 2013 | 3 | 100 | 0.33 | Abatacept | 16.2 |
| 2. AVERT18 | 2015 | 4 | 235 | 1.00 | Abatacept | 15–20* |
| 3. PREMIER15 | 2006 | 4 | 542 | 2.00 | Adalimumab | 20 |
| 4. ADORE30 | 2006 | 2 | 314 | 0.31 | Etanercept | ≥15 |
| 5. COMET17 | 2010 | 5 | 312 | 1.00 | Etanercept | 17 |
| 6. Iannone21 | 2014 | 3 | 20 | 1.04 | Etanercept | 10 |
| 7. JESMR22 | 2011 | 2 | 147 | 1.00 | Etanercept | 7 |
| 8. TEMPO29 | 2006 | 4 | 454 | 3.00 | Etanercept | 16.4 |
| 9. GO-BEFORE17 | 2013 | 5 | 637 | 2.00 | Golimumab | 19.15 |
| 10. GO-FORWARD24 | 2013 | 5 | 444 | 2.00 | Golimumab | 15–25 |
| 11. Taylor28 | 2011 | 4 | 514 | 0.92 | Golimumab | 15–25 |
| 12. Maini25 | 1998 | 2 | 87 | 0.50 | Infliximab | 7.5 |
| 13. Edwards16 | 2004 | 4 | 80 | 0.92 | Rituximab | ≥10 |
| 14. ACT-RAY20 | 2014 | 5 | 553 | 2.00 | Tocilizumab | ≥15 |
| 15. CHARISMA26 | 2006 | 5 | 310 | 0.38 | Tocilizumab | 10–25 |
| 16. SURPRISE23 | 2016 | 3 | 226 | 1.00 | Tocilizumab | 8 |
*Possibility of changing to a dose <10 mg/week in the case of intolerance.
bDMARDs, biologic disease-modifying antirheumatic drugs; MTX, methotrexate.
Characteristics of the populations studied
The studies were homogeneous for the criteria for inclusion in the trials, study population characteristics and concomitant medications (see online supplementary table S2 in appendix 2).
Risk of the occurrence of serious adverse events
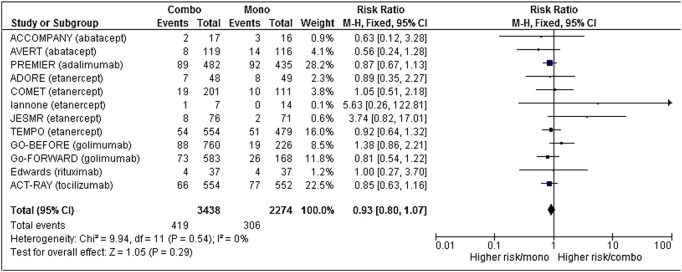
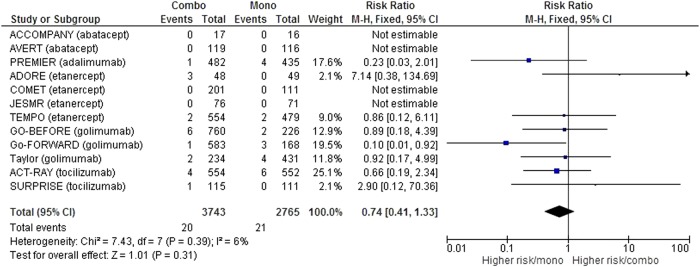
The number of serious adverse events was available for 12 studies,15–22 24 27 29 30 corresponding to 5712 patient-years, which was 3438 in the combination group and 2274 in the monotherapy group.
After meta-analysis, the risk of serious adverse events was comparable in both groups (RR 0.92 (95% CI (0.78 to 1.08)) (figure 2), but with a trend in favour of the combination group. Details of serious adverse events when they were available are summarised in online supplementary table S3 (appendix 3).
Figure 2.
Forest plot comparing the risk of serious adverse events between the combination therapy and monotherapy groups.
Risk of the occurrence of serious infections
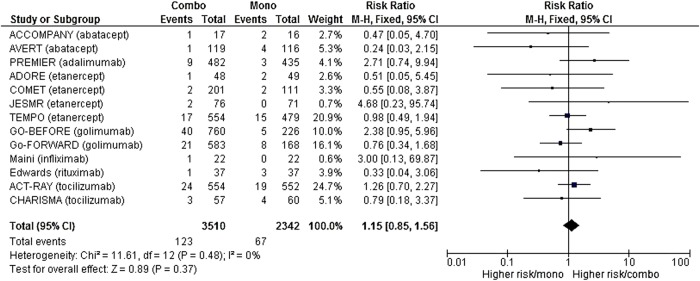
The number of serious infections was available for 13 studies,15–20 22 24–27 29 30 corresponding to 5852 patient-years, which was 3510 in the combination group and 2342 in the monotherapy group.
After meta-analysis, no difference was identified in serious infection risk between the two groups (RR 1.15 (95% CI (0.84 to 1.58)) (figure 3). Details of serious infections are available in online supplementary table S3 (appendix 3).
Figure 3.
Forest plot comparing the risk of serious infections between the combination therapy and monotherapy groups.
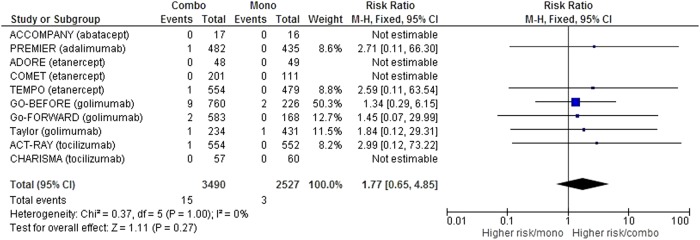
Regarding the risk of tuberculosis, we collected data in 10 studies,15 17 19 20 24 26–30 or 6017 patient-years, which was 3490 in the combination group and 2527 in the monotherapy group. Screening procedures for latent tuberculosis when they were described are detailed in online supplementary table S4 (appendix 4). After meta-analysis, no significant difference was found in the risk of tuberculosis among the groups (RR=1.78 (95% CI (0.63 to 4.99)), but we noted a trend towards a high risk in the combination group (figure 4) with 15 cases of tuberculosis on 3490 patient-years in the combination group compared with only 3 cases of tuberculosis on 2527 patient-years in the monotherapy group.
Figure 4.
Forest plot comparing the risk of tuberculosis between the combination therapy and monotherapy groups.
Risk of death
We collected the number of deaths in 12 studies15 17–20 23 24 26–30 or 6508 patient-years, which was in 3743 in the combination group and 2725 in the monotherapy group.
After meta-analysis, no significant difference was found in mortality between the groups (RR 0.73 (95% CI (0.40 to 1.35) (figure 5).
Figure 5.
Forest plot comparing the risk of death between the combination therapy and monotherapy groups.
All causes of death in these studies were detailed including 20 in the combination group and 21 in the monotherapy group (see online supplementary table S3 for details of death in appendix 3).
Twenty per cent of deaths in the combination group were related to an infectious cause compared with 14.3% in the monotherapy group. All cardiac causes (cardiac arrest, heart failure, arrhythmia, myocardial infarction) represented 25% of causes of death of patients in the combination group compared with 28.6% of patients on monotherapy. Finally, four deaths were from cancer in the combination group (20%) and two in the monotherapy group (9.5%).
Discussion
We did not show any significant difference between the occurrence of serious adverse events, serious infections, tuberculosis or death in patients under methotrexate and bDMARD combination therapy compared with bDMARD monotherapy in RA.
The originality of our work is that, to the best of our knowledge, there are no published meta-analyses comparing the safety of monotherapy versus bDMARDs prescribed in combination with methotrexate.
The meta-analysis by Singh et al published in 201511 concluded that the use of standard dose bDMARDs (alone or in combination with csDMARDs) in RA was associated with an increase in serious infections compared with csDMARDs only: RR=1.31 (95% CI (1.09 to 1.58)). This study compared bDMARDs with csDMARDs, but did not study the difference in risk between bDMARDs prescribed in combination with csDMARDs and in monotherapy as we did. It therefore does not provide the same conclusions as our analysis.
In the American Registry CORRONA (Consortium of Rheumatology Researchers of North America),8 of 7971 patient-years followed, there was an infection rate of 40.1/100 PY (95% CI (37.0 to 43.4 )) under anti-TNF-α and 37.1/100 PY (95% CI (34.9 to 39.3)) under the anti-TNF-α and methotrexate combination therapy. Similarly, in the Spanish register BIOBADASER,31 the use of methotrexate did not appear as a factor associated with serious infections.
Regarding the specific risk of developing tuberculosis, we did not show any significant difference between combination therapy and monotherapy, but there was a trend towards higher risk in the combination group. The low number of events in each trial possibly explains the lack of significance of the meta-analysis. This trend is similar to a study by Lorenzetti et al published in 2014.12 This systematic literature review showed that the risk of tuberculosis under anti-TNF-α therapy was higher when these agents were used in combination with methotrexate than in monotherapy with an RR of 13.3 (95% CI (3.7 to 100)). However, this work took into account the use of anti-TNF-α in rheumatic diseases (RA, ankylosing spondylitis, psoriatic arthritis) and also in skin (psoriasis) and digestive (chronic inflammatory bowel) diseases. Moreover, it did not take into account all bDMARDs, but rather only a few anti-TNF-α therapies (infliximab, adalimumab and certolizumab). In addition, csDMARDs prescribed in combination can be methotrexate, as well as azathioprine. Finally, the limits of the CI are very wide, which reduces the accuracy of the OR observed.
The strengths of our study are that it is first of all a systematic review of the literature with meta-analysis, taking into account randomised controlled trials. The selected studies had good methodological quality with a Jadad score=3/5 for 13 of them. Furthermore, a full reading of articles and data collection was performed independently by two individuals. Finally, the trials included in this meta-analysis were homogeneous, which reinforces even more the results obtained. The key words in our search strategy included ‘randomized controlled trial’ and trials not indexed as ‘randomized clinical trials’ in PubMed might have been missed. However, our search strategy search was also based on Embase and Cochrane CENTRAL and not restricted to randomised controlled trials in these databases. Another potential limitation of this work is that our review was based on only 16 trials and the results might be different with more studies. However, the trials were homogeneous with narrow CIs; thus, we assume that additional trials would most likely not change the direction of the results.
Our work reports in RA no significant difference in the occurrence of serious adverse events, serious infections, tuberculosis or death between bDMARDs as monotherapy compared with bDMARDs and methotrexate. This confirms the safety of the established choice to combine methotrexate with a bDMARD, in the absence of methotrexate contraindication or intolerance, without any additional risk to the patient. However, we note a trend, although insignificant, in favour of monotherapy for the risk of tuberculosis.
Acknowledgments
The authors are very grateful to the professors and organisers of ASLER seminary (for Systematic Analysis of the Literature in Rheumatology) for their useful advice in the writing of this manuscript. The authors wish to thank Abbott who provided logistic support in the organisation of sessions about the implementation of a meta-analysis, and remained independent of the collection, analysis and interpretation of data.
Footnotes
Contributors: CB and AR-W equally contributed to the conception of the study, the article selection process, the data collection, the data analysis, the results interpretation, the manuscript writing and approval. YD, AC and AlC contributed to the conception of the study, results interpretation and manuscript approval. All the authors take responsibility for the integrity of the work as a whole, from inception to published article, and they should indicate that they had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. They give permission to reproduce published material, report sensitive personal information, use illustrations of identifiable persons or name persons for their contributions.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The Ethics Committee approvals of each trial were obtained for all the studies selected in this meta-analysis.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: This study involved only published reports and no unpublished information. No database was used for this study.
References
- 1.Smolen JS, Landewé R, Breedveld FC et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaujoux-Viala C, Nam J, Ramiro S et al. . Efficacy of conventional synthetic disease-modifying antirheumatic drugs, glucocorticoids and tofacitinib: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2014;73:510–15. 10.1136/annrheumdis-2013-204588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nam JL, Ramiro S, Gaujoux-Viala C et al. . Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2014;73:516–28. 10.1136/annrheumdis-2013-204577 [DOI] [PubMed] [Google Scholar]
- 4.Doran MF, Crowson CS, Pond GR et al. . Predictors of infection in rheumatoid arthritis. Arthritis Rheum 2002;46:2294–300. 10.1002/art.10529 [DOI] [PubMed] [Google Scholar]
- 5.Doran MF, Crowson CS, Pond GR et al. . Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 2002;46:2287–93. 10.1002/art.10524 [DOI] [PubMed] [Google Scholar]
- 6.Franklin J, Lunt M, Bunn D et al. . Risk and predictors of infection leading to hospitalisation in a large primary-care-derived cohort of patients with inflammatory polyarthritis. Ann Rheum Dis 2007;66:308–12. 10.1136/ard.2006.057265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford) 2013;52:53–61. 10.1093/rheumatology/kes305 [DOI] [PubMed] [Google Scholar]
- 8.Greenberg JD, Reed G, Kremer JM et al. . Association of methotrexate and tumour necrosis factor antagonists with risk of infectious outcomes including opportunistic infections in the CORRONA registry. Ann Rheum Dis 2010;69:380–6. 10.1136/ard.2008.089276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLean-Tooke A, Aldridge C, Waugh S et al. . Methotrexate, rheumatoid arthritis and infection risk: what is the evidence? Rheumatology (Oxford) 2009;48:867–71. 10.1093/rheumatology/kep101 [DOI] [PubMed] [Google Scholar]
- 10.van der Veen MJ, van der Heide A, Kruize AA et al. . Infection rate and use of antibiotics in patients with rheumatoid arthritis treated with methotrexate. Ann Rheum Dis 1994;53:224–8. 10.1136/ard.53.4.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh JA, Cameron C, Noorbaloochi S et al. . Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet 2015;386:258–65. 10.1016/S0140-6736(14)61704-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenzetti R, Zullo A, Ridola L et al. . Higher risk of tuberculosis reactivation when anti-TNF is combined with immunosuppressive agents: a systematic review of randomized controlled trials. Ann Med 2014;46:547–54. 10.3109/07853890.2014.941919 [DOI] [PubMed] [Google Scholar]
- 13.Ruderman EM. Overview of safety of non-biologic and biologic DMARDs. Rheumatology (Oxford) 2012;51(Suppl 6):vi37–43. 10.1093/rheumatology/kes283 [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breedveld FC, Weisman MH, Kavanaugh AF et al. . The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. 10.1002/art.21519 [DOI] [PubMed] [Google Scholar]
- 16.Edwards JC, Szczepanski L, Szechinski J et al. . Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004;350:2572–81. 10.1056/NEJMoa032534 [DOI] [PubMed] [Google Scholar]
- 17.Emery P, Breedveld F, van der Heijde D et al. . Two-year clinical and radiographic results with combination etanercept-methotrexate therapy versus monotherapy in early rheumatoid arthritis: a two-year, double-blind, randomized study. Arthritis Rheum 2010;62:674–82. 10.1002/art.27268 [DOI] [PubMed] [Google Scholar]
- 18.Emery P, Burmester GR, Bykerk VP et al. . Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis 2015;74:19–26. 10.1136/annrheumdis-2014-206106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emery P, Fleischmann RM, Doyle MK et al. . Golimumab, a human anti-tumor necrosis factor monoclonal antibody, injected subcutaneously every 4 weeks in patients with active rheumatoid arthritis who had never taken methotrexate: 1-year and 2-year clinical, radiologic, and physical function findings of a phase III, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res (Hoboken) 2013;65:1732–42. 10.1002/acr.22072 [DOI] [PubMed] [Google Scholar]
- 20.Huizinga TW, Conaghan PG, Martin-Mola E et al. . Clinical and radiographic outcomes at 2 years and the effect of tocilizumab discontinuation following sustained remission in the second and third year of the ACT-RAY study. Ann Rheum Dis 2015;74:35–43. 10.1136/annrheumdis-2014-205752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iannone F, La Montagna G, Bagnato G et al. . Safety of etanercept and methotrexate in patients with rheumatoid arthritis and hepatitis C virus infection: a multicenter randomized clinical trial. J Rheumatol 2014;41:286–92. 10.3899/jrheum.130658 [DOI] [PubMed] [Google Scholar]
- 22.Kameda H, Kanbe K, Sato E et al. . Continuation of methotrexate resulted in better clinical and radiographic outcomes than discontinuation upon starting etanercept in patients with rheumatoid arthritis: 52-week results from the JESMR study. J Rheumatol 2011;38:1585–92. 10.3899/jrheum.110014 [DOI] [PubMed] [Google Scholar]
- 23.Kaneko Y, Atsumi T, Tanaka Y et al. . Comparison of adding tocilizumab to methotrexate with switching to tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52-week results from a prospective, randomised, controlled study (SURPRISE study). Ann Rheum Dis 2016;75:1917–23. 10.1136/annrheumdis-2015-208426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keystone EC, Genovese MC, Hall S et al. . Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: results through 2 years of the GO-FORWARD study extension. J Rheumatol 2013;40:1097–103. 10.3899/jrheum.120584 [DOI] [PubMed] [Google Scholar]
- 25.Maini RN, Breedveld FC, Kalden JR et al. . Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum 1998;41:1552–63. [DOI] [PubMed] [Google Scholar]
- 26.Maini RN, Taylor PC, Szechinski J et al. . Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum 2006;54:2817–29. 10.1002/art.22033 [DOI] [PubMed] [Google Scholar]
- 27.Nash P, Nayiager S, Genovese MC et al. . Immunogenicity, safety, and efficacy of abatacept administered subcutaneously with or without background methotrexate in patients with rheumatoid arthritis: results from a phase III, international, multicenter, parallel-arm, open-label study. Arthritis Care Res (Hoboken) 2013;65:718–28. 10.1002/acr.21876 [DOI] [PubMed] [Google Scholar]
- 28.Taylor PC, Ritchlin C, Mendelsohn A et al. . Maintenance of efficacy and safety with subcutaneous golimumab among patients with active rheumatoid arthritis who previously received intravenous golimumab. J Rheumatol 2011;38:2572–80. 10.3899/jrheum.110570 [DOI] [PubMed] [Google Scholar]
- 29.van der Heijde D, Klareskog L, Rodriguez-Valverde V et al. . Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum 2006;54:1063–74. 10.1002/art.21655 [DOI] [PubMed] [Google Scholar]
- 30.van Riel PL, Taggart AJ, Sany J et al. . Efficacy and safety of combination etanercept and methotrexate versus etanercept alone in patients with rheumatoid arthritis with an inadequate response to methotrexate: the ADORE study. Ann Rheum Dis 2006;65:1478–83. 10.1136/ard.2005.043299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cobo-Ibáñez T, Descalzo MÁ, Loza-Santamaría E et al. . Serious infections in patients with rheumatoid arthritis and other immune-mediated connective tissue diseases exposed to anti-TNF or rituximab: data from the Spanish registry BIOBADASER 2.0. Rheumatol Int 2014;34:953–61. 10.1007/s00296-014-2945-y [DOI] [PubMed] [Google Scholar]