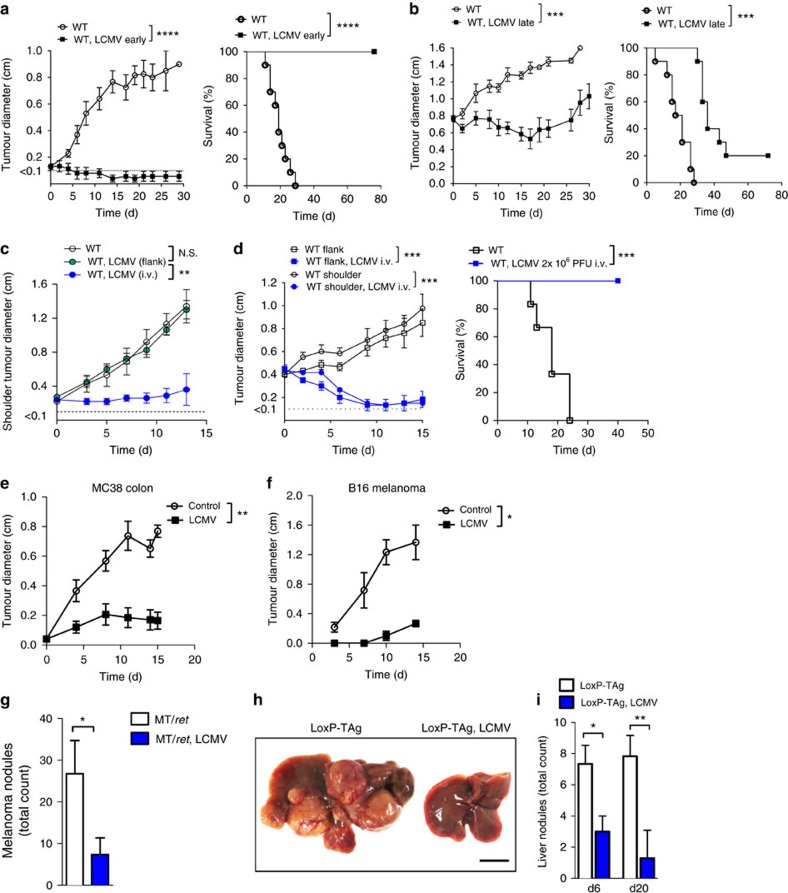
Figure 2. Persistence of arenavirus leads to tumour regression.
(a) Tumour diameter and survival of MOPC-tumour-bearing mice (day −3) treated with or without 2 × 104 PFU LCMV peritumourally (n=10 per group, three experiments pooled). (b) Tumour diameter and survival of MOPC-tumour-bearing mice (day −10) treated with or without 2 × 104 PFU LCMV intratumourally (n=10 per group, three experiments pooled). (c) Tumour diameter of the shoulder tumour from C57BL/6 mice receiving simultaneously subcutaneously 5 × 105 MOPC cells in the flank and shoulder (day −3), treated without (n=5) or with 2 × 104 PFU LCMV given into the flank (n=4) or intravenously (n=5). (d) Tumour diameters (shoulder and flank) and survival of WT mice bearing MOPC tumours simultaneously in the shoulder and flank, treated with or without 2 × 106 PFU LCMV intravenously on day 0 (n=6 per group). (e) Tumour diameter from MC38-bearing C57BL/6 mice (day −3) treated with (n=6) or without (n=7) 2 × 104 PFU LCMV peritumourally. (f) Tumour diameters from B16F10-tumour-bearing C57BL/6 mice (day −3) treated with or without 2 × 104 PFU LCMV peritumourally (n=6 per group). (g) Number of melanomas (day 15) in MT/ret mice treated with (n=3) or without (n=4) 2 × 104 PFU LCMV systemically. (h,i) Representative picture (h, day 6, n=3) and quantification of tumour nodules (i, day 6 n=3, day 20 n=6) of/in the livers from LoxP-Tag-tumour-bearing or WT mice, which were treated with or without 2 × 106 PFU LCMV systemically. Scale bar, 0.5 cm. Data are shown as mean±s.e.m. and analysed by unpaired Student's t-test. *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001.