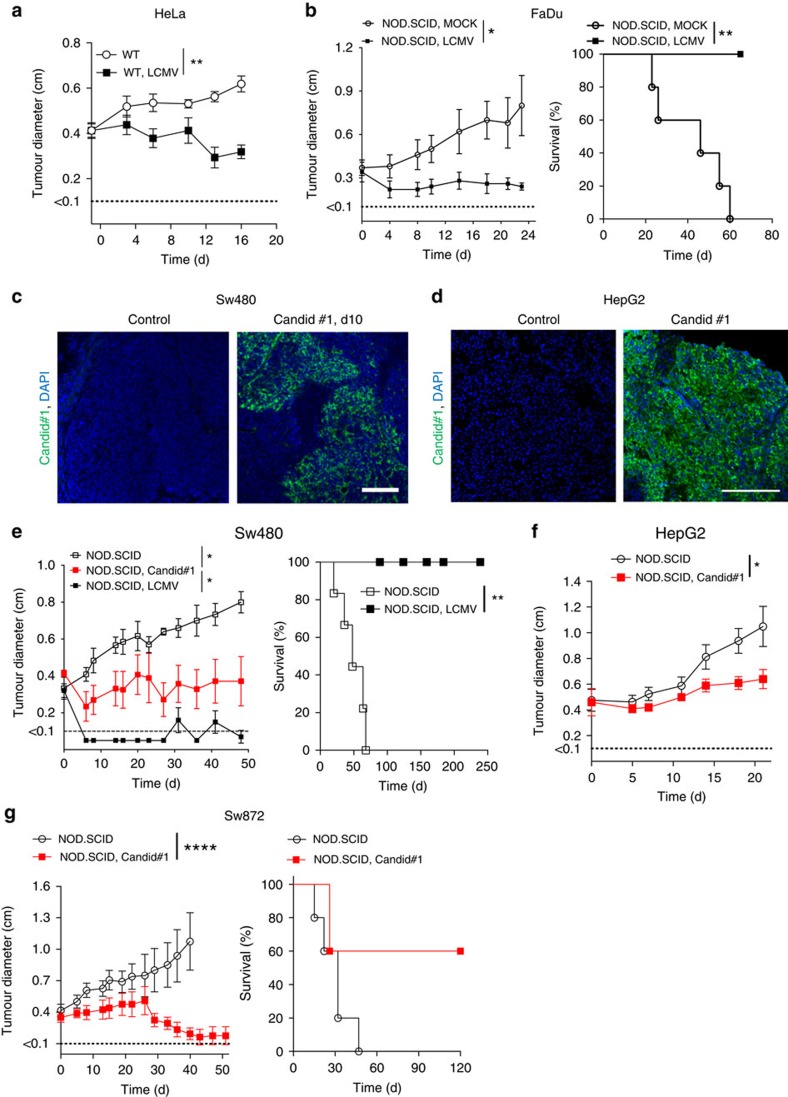
Figure 5. LCMV and arenavirus vaccine Candid#1 induce regression of human tumours.
(a) Tumour diameter from HeLa-tumour-bearing NOD/SCID mice (day −8) treated with or without 2 × 104 PFU LCMV intratumourally (n=8 per /group). (b) Tumour diameter and survival from FaDu-tumour-bearing NOD/SCID mice (day −10) treated with or without 2 × 106 PFU LCMV i.t. (n=5 per group) (c) Immunofluorescence (day 10) of tumours from Sw480-tumour-bearing NOD/SCID mice (day −10) treated with or without 2 × 104 PFU Candid#1 intratumoural (n=3 per group). Scale bar, 200 μm. (d) Immunofluorescence (day 10, n=3 per group) from HepG2-tumour-bearing NOD/SCID mice treated with or without 5 × 105 PFU Candid#1 intratumourally. Scale bar, 200 μm. (e) Tumour diameter and survival from Sw480-tumour-bearing NOD/SCID mice (day −11) treated with (n=5) or without (n=6) 5 × 105 PFU LCMV or 2 × 104 PFU Candid#1 (n=7) intratumourally. (f) Tumour diameter from HepG2-tumour-bearing NOD/SCID mice treated with (n=5) or without (n=4) 5 × 105 PFU Candid#1 intratumourally. (g) Tumour diameter (untreated n=6; treated n=8) and survival (n=5 per group) from Sw872 liposarcoma-bearing NOD/SCID mice treated with or without 5 × 105 PFU Candid#1. Data are shown as mean±s.e.m. and analysed by unpaired Student's t-test. Survival is shown in Kaplan–Meier method and analysed by log-rank test. *P<0.05, **P<0.01 and ****P<0.0001