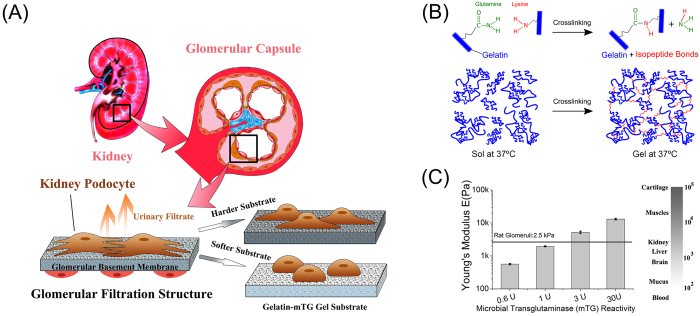
Figure 1. Stiffness-tunable gelatin-mTG hydrogel provides an ideal platform to study kidney podocyte mechanotransduction.
(A) Schematic representation of human podocyte study using tunable stiffness gels to characterize podocyte phenotype. The cross-section of a kidney and its main functional unit, i.e. the glomerular capsule are shown (top). The tissue structure inside the glomerular capsule is framed to highlight the location of the glomerular filtration barrier, for which podocytes are essential. Podocytes (in brown) interdigitate with neighboring podocytes to form the slit diaphragm. The glomerular basement membrane provides mechanical support for podocytes. Capillary endothelial cells (in red) are separated from podocytes by the basement membrane. Mutations in glomerular basement membrane components and physical structure are found in many nephropathies, which are correlated with changes in glomerular tissue stiffness. By using a gel system with tunable stiffness, podocyte mechanotransduction can be analyzed. (B) Illustration of (top) the transglutamination reaction used for gelatin crosslinking, and (bottom) a schematic of the resulting crosslinked gelatin network. Microbial transglutaminase initiates the formation of covalent isopeptide bonds between lysine and glutamine residues on gelatin molecules. (C) Young’s moduli of gelatin-mTG gels crosslinked by four enzyme concentrations (0.6U, 1U, 3U and 30U) as measured by oscillatory rheology. At right, a scale demonstrating tissue stiffness of various human organs and soft tissues is shown.