Fig. 2.
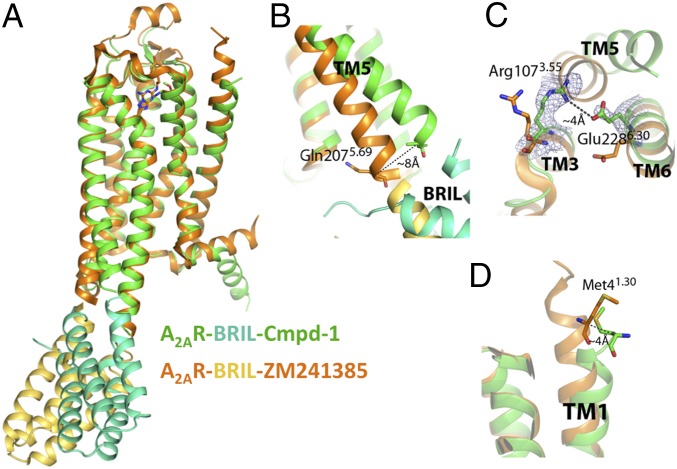
Structure features of A2AR–BRIL in complex with Cmpd-1. (A) Overall structure comparison of A2AR–BRIL–Cmpd-1 (receptor and ligand in green; BRIL in cyan) and A2AR–BRIL–ZM241385 (receptor and ligand in orange; BRIL in gold). (B) Structural comparison of the intracellular part of TM5. In the A2AR–BRIL–Cmpd-1 structure, TM5 is displaced by 8 Å at the Cα of Gln2075.69 relative to the A2AR–BRIL–ZM241385 structure. (C) In the A2AR–BRIL–Cmpd-1 structure, Glu2286.30 forms a salt bridge with Arg1073.55, which is only possible because of the displacement of TM5 noted above. The map is 2Fo–Fc map contoured at 1.5 σ. (D) Structural comparison of the N-terminal aspect of the TM1 helices between the two structures where the Cα atoms of Met41.30 are ∼4 Å apart.