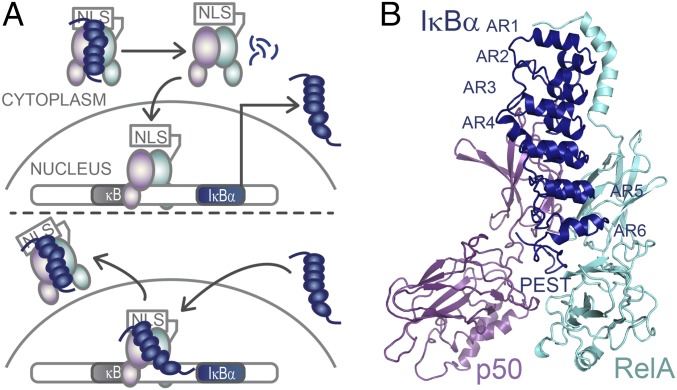
Fig. 1.
IκBα regulates NFκB activity. (A) IκBα sequesters NFκB in the cytoplasm. When an extracellular stress signal (e.g., LPS, TNFα) is received, IκBα is phosphorylated by IKK, ubiquitinated, and degraded, thus revealing the NFκB nuclear localization signal (NLS), whereupon it enters the nucleus and binds to κB DNA sites. One of the genes under control of the κB promoter is IκBα, so newly synthesized IκBα then enters the nucleus, strips NFκB from DNA, and exports NFκB out of the nucleus. (B) Structural model of NFκB (RelA/p50) bound to the six-AR–containing IκBα showing the C-terminal PEST sequence [Protein Data Bank (PDB) 1IKN–1VKX composite] (25).