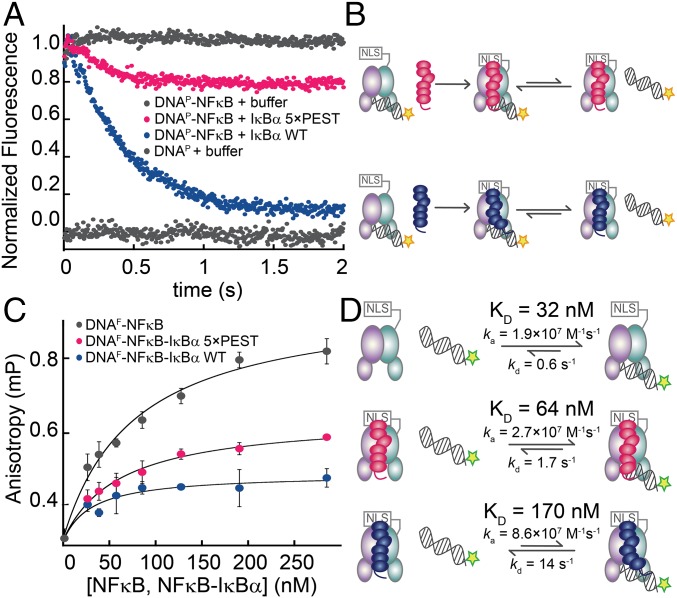
Fig. 2.
The IκBα(5xPEST) mutant formed a stabilized NFκB–DNA–IκBα ternary complex. (A) Stopped-flow fluorescence traces corresponding to the dissociation of pyrene-labeled DNA (DNAP) from NFκB. A fivefold excess of IκBα(WT) rapidly strips DNAP from NFκB, whereas a fivefold excess of IκBα(5xPEST) retained bound DNAP. For reference, traces of NFκB–DNAP and DNAP injected against buffer are shown at normalized fluorescence values of 1 and 0, respectively. (B) Schematic showing the reactions being monitored by the stopped-flow fluorescence experiment. The pyrene on DNAP is indicated by a yellow star. (C) Equilibrium binding affinities were determined by monitoring the increase in steady-state fluorescence anisotropy of FITC-labeled DNA (DNAF) as a function of the concentration of NFκB, NFκB–IκBα(5xPEST), or NFκB–IκBα(WT). (D) Schematic highlighting the rates and equilibrium binding affinities of DNA with NFκB, NFκB–IκBα(5xPEST), and NFκB–IκBα(WT). The stripping-impaired IκBα(5xPEST) complex shifts the equilibrium toward the ternary NFκB–DNA–IκBα complex. The fluorescein on DNAF is indicated by a green star.