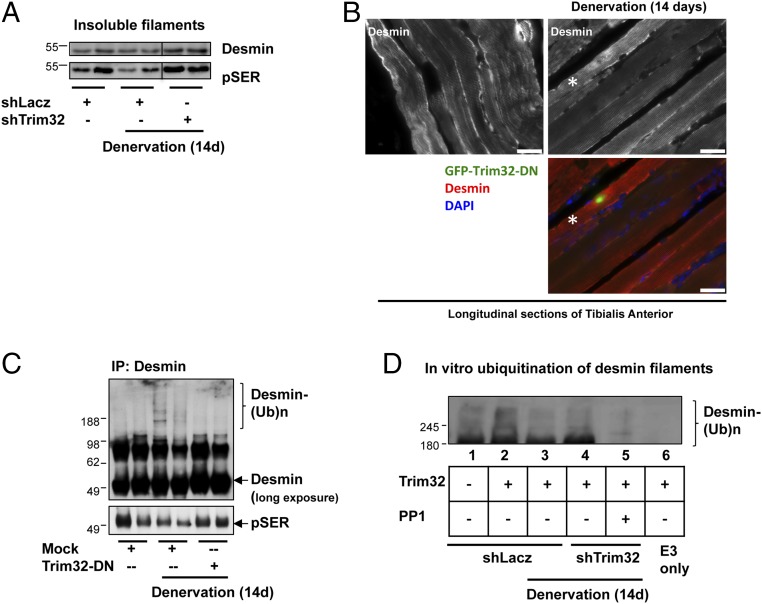
Fig. 5.
On denervation, Trim32 catalyzes the loss of phosphorylated desmin filaments. (A) Trim32 promotes the disassembly and degradation of phosphorylated desmin filaments after denervation. Desmin filaments were isolated from innervated and denervated muscles expressing shLacz or shTrim32 and then analyzed by SDS/PAGE and immunoblotting. Phosphorylated desmin was detected with anti-phosphoserine antibody. (B) Desmin is degraded in atrophy induced by denervation. Paraffin-embedded longitudinal sections of innervated and denervated TA muscles expressing Trim32-DN-GFP were stained with an antibody against desmin. Asterisk indicates transfected fiber expressing GFP-Trim32-DN. (Scale bar: 35 µm.) (C) On denervation, desmin is ubiquitinated, but when Trim32 is inhibited, phosphorylated species accumulate in the cytosol. Desmin was immunoprecipitated from soluble fractions of control or denervated muscles expressing shLacz or Trim32-DN. Precipitates were analyzed by immunoblotting using antibodies against desmin and phosphoserine. (D) On denervation, phosphorylation of desmin filaments facilitates their ubiquitination by Trim32. Isolated desmin filaments from normal muscles (lanes 1 and 2) and atrophying muscles (lanes 3–5) expressing shLacz (lanes 1–3) or shTrim32 (lanes 4 and 5) were treated with protein phosphatase 1 (PP1; lane 5) or left untreated (lanes 1–4), and then subjected to ubiquitination by recombinant Trim32 and UbcH5 using 6His-tagged ubiquitin. Ubiquitinated desmin was purified with a nickel column and analyzed by SDS/PAGE and immunoblotting using anti-desmin.