Fig. 5.
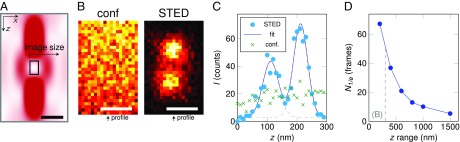
MINFIELD is especially advantageous for STED imaging along the optic axis (z) and in 3D. (A) The focal STED intensity distribution for confining molecular fluorescence along the optic axis (z) and hence in 3D extends well over >1 μm in z. Achieving similar resolution as in the focal plane requires higher laser intensities. (B) Three-dimensional imaging is demonstrated on Atto647N-labeled DNA origami with two spots designed to be separated by 91 nm. The resolution improvement with STED is immediately apparent when comparing the confocal xz image with its MINFIELD STED counterpart. (C) Analyzing the line profile along the z axis yields a peak-to-peak separation of ∼100 nm at a full width at half-maximum of ∼60 nm for the Gaussian fits. (D) Number of image frames that could be acquired before the fluorescence signal dropped below 1/e of its original brightness, depending on the scan range along the z axis. The data were acquired by imaging antibody-labeled microtubules in an x–z scan, using a “top-hat”–only phase pattern and keeping the x-range constant at 5 μm (see data in Fig. 3 B and C, where the imaged fields were squares, and, consequently, the relative area reductions stronger than for the data shown here). The indicated z range (dashed line) corresponds to the size of the images in B. [Scale bars: 500 nm (A), 100 nm (B).]