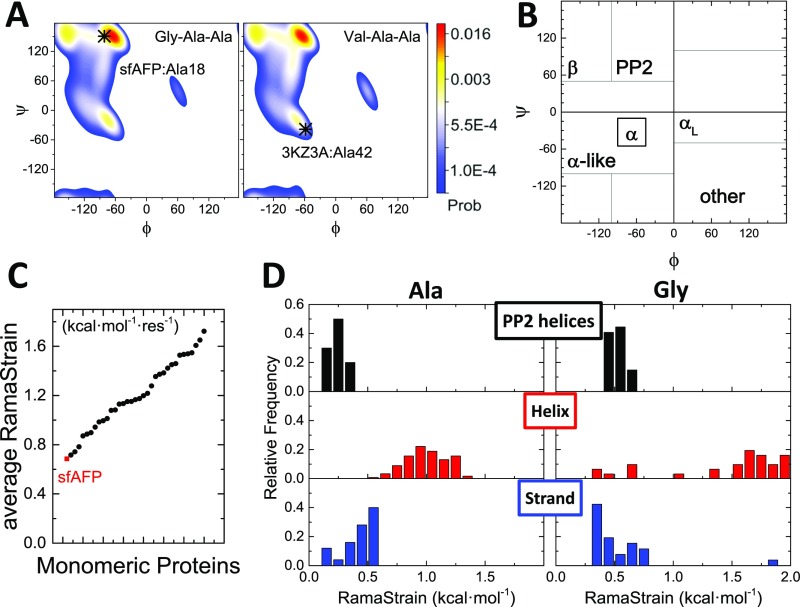
Fig. S4.
RamaStrain. (A) Ramachandran probability landscapes for Ala18 and Ala42 in the unfolded states of sfAFP and λ-repressor, respectively, based on a coil library (59). (B) Ramachandran basin boundaries used in the RamaStrain calculation. (C) RamaStrain was evaluated for a set of 34 proteins of comparable size to sfAFP. The average RamaStrain in sfAFP is lower than for similarly sized proteins. (D) Distributions of RamaStrain for Ala and Gly residues in sfAFP and the test set, organized by secondary structure. A bias against helices (and, to a lesser extent, strands) in the unfolded state is apparent.