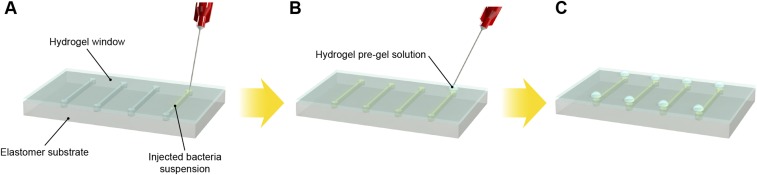
Fig. S1.
Schematic illustration of cell suspension injection and sealing of injection points. (A) Bacteria were injected into the cavities at the hydrogel–elastomer interface with metallic needles from the hydrogel side. (B) Injection holes were sealed on the hydrogel–elastomer device with drops of fast-curable pregel solution. (C) We obtained the hydrogel–elastomer device with fully encapsulate bacteria.