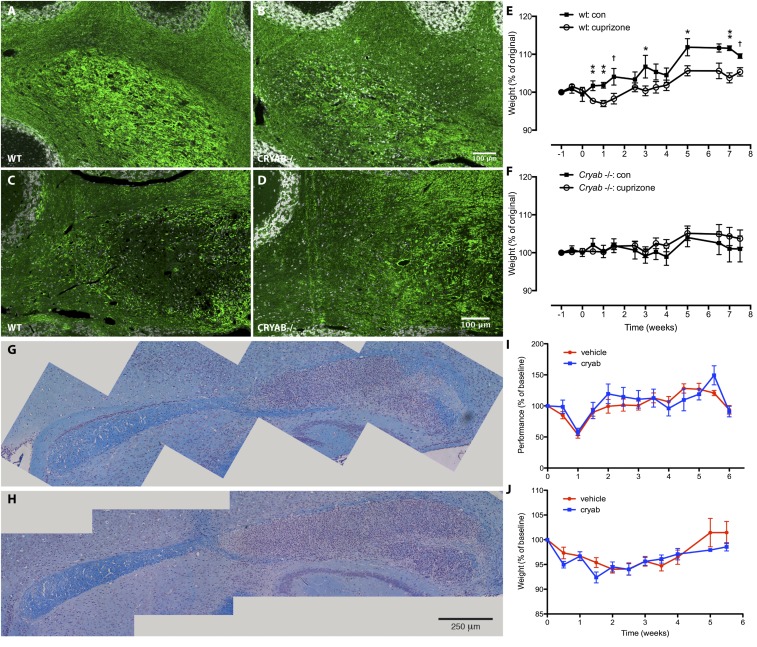
Fig. S1.
CRYAB expression determines severity of cuprizone-induced symptoms, but treatment with exogenous CRYAB does not affect severity of cuprizone-induced demyelinating pathology. (A–D) Immunofluorescence staining for myelin basic protein (MBP) in cerebellar tissue of mice treated with cuprizone for 6 (A and B) or 8 wk (C and D) shows that cuprizone-induced demyelination is more severe in WT mice (A and C) than in Cryab−/− mice (B and D). Myelin damage is visible in WT mice after 6 wk of cuprizone treatment (as increased availability of MBP antigen; A), whereas myelin loss is prominent after 8 wk (C). (E and F) Assessing body weight as a measure of clinical outcome of cuprizone-induced pathology shows that WT mice (F) are more susceptible to cuprizone-induced symptoms than in Cryab−/− mice (G). Data shown are mean ± SEM and P values determined by unpaired t test (n = 4–8; †P < 0.1, *P < 0.05, **P < 0.01). (G and H) LFB staining of myelin in corpus callosum of WT C57/Bl6 mice treated with vehicle (A) or CRYAB (B; 10 µg, i.p., every other day), showing extensive demyelination and inflammation in the caudal region of the corpus callosum. (I and J) Motor skill performance (C) and body weight (D) as outcome measures of cuprizone-induced pathology, reveal that clinical outcome is not different between mice treated with vehicle (red) or CRYAB (blue). Data shown are mean ± SEM (n = 4).