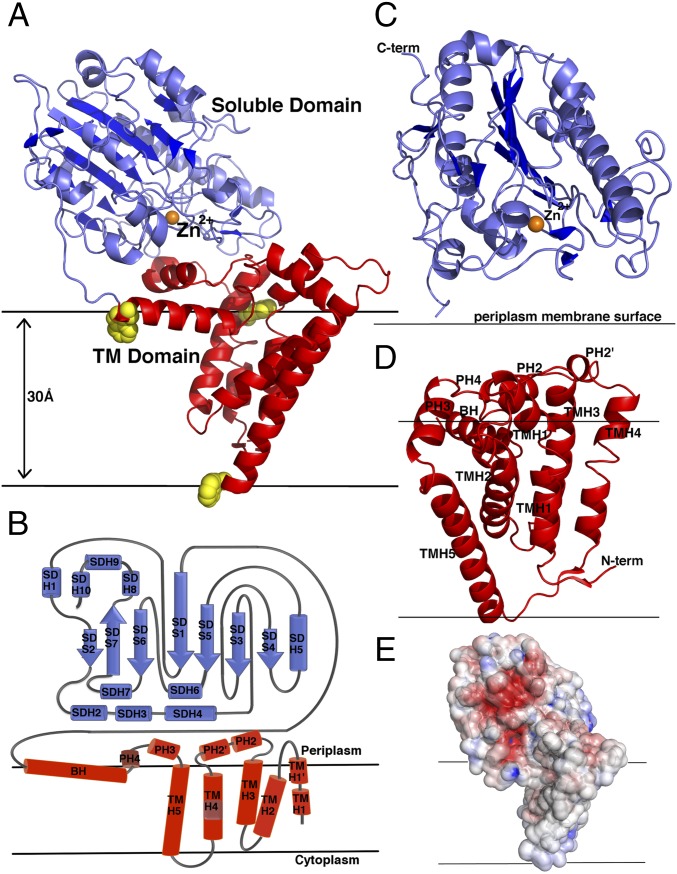
Fig. 1.
Molecular structure of NmEptA. (A) Ribbon representation of NmEptA. The amino-terminal TM domain is shown in red, and the carboxyl-terminal soluble domain is shown in blue. The side chains of the three TM domain tryptophan residues (Trp126, Trp148, and Trp207) are shown as yellow spheres. (B) The topology diagram of NmEptA showing the likely positioning of the protein relative to the periplasmic membrane with the coloring as shown in A. (C) Secondary structure of the soluble domain and (D) TM domain with the helical numbering labeled. (E) Electrostatic surface representation of NmEptA, calculated using APBS. The surface is color-contoured from −4 kT/e to +4 kT/e (negative in red, positive in blue). The bound Zn2+ ion in A and C is shown as an orange sphere. To delineate the proposed orientation of the protein within the bilayer, the membrane surface, representing the hydrophobic portion of the bilayer, is drawn as horizontal black lines.