Figure 3.
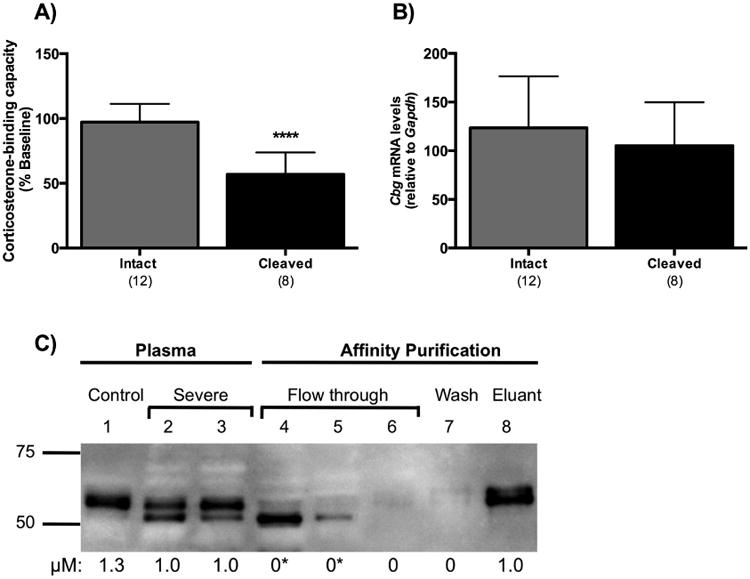
Proteolysis of CBG is associated with reduced plasma CBG levels without changes in liver Cbg mRNA levels, and evidence that cleaved CBG in plasma lacks steroid-binding activity. (A) Rats with cleaved CBG had significantly lower plasma CBG values than rats with intact CBG. (B) Liver Cbg mRNA levels were similar irrespective of CBG proteolysis status. In A and B, samples were grouped for analysis based on CBG integrity, as assessed by western blotting (see Figure 2C), and classified as being either intact or cleaved. The numbers of animals in each group are shown in parentheses, and data are presented as mean ± SD. **** P < 0.0001. (C) Plasma from rats with severe inflammation (lanes 2 and 3) were pooled and purified by steroid affinity chromatography. Cleaved CBG did not bind the steroid-affinity matrix and eluted in the flow through (lanes 4 and 5). Intact CBG bound to the steroid-affinity matrix and was eluted with buffer containing excess corticosterone (lane 8). CBG-corticosterone binding capacity values (μM) are shown under each lane. There was no detectable CBG steroid-binding activity (*) in the flow through fractions that contained cleaved CBG (lanes 4 and 5). Intact CBG (lane 8) exhibited full steroid-binding activity. An intact control (saline) sample is shown (lane 1) for comparison.
