Abstract
Background
Higher body mass index (BMI) is a risk factor for cardiovascular disease, including heart failure. Obesity disproportionately affects African-Americans; however, the association between higher BMI and left ventricular (LV) function in African-Americans is not well understood.
Objectives
To assess whether BMI is associated with subclinical LV systolic dysfunction in African-American individuals.
Methods
In 1,652 adult African-American participants of the Jackson Heart Study peak systolic circumferential strain (Ecc) was measured by tagged cardiac MRI between 2008-2012. We evaluated the association between BMI and Ecc in multivariable linear regression and restricted cubic spline analyses adjusted for prevalent cardiovascular disease, traditional cardiovascular risk factors, LV mass, and ejection fraction. In exploratory analyses, we also examined whether inflammation, insulin resistance, or volume of visceral adipose tissue altered the association between BMI and Ecc.
Results
The proportions of female, non-smokers, diabetic, and hypertensive participants rose with increase in BMI. In multivariable-adjusted models, higher BMI was associated with worse Ecc, (β=0.052; 95% CI: 0.028, 0.075), even in the setting of preserved LV ejection fraction. Higher BMI was also associated with worse Ecc when accounting for markers of inflammation (CRP, E-selection, P-selectin), insulin resistance, and volume of visceral adipose tissue.
Conclusions
Higher BMI is significantly associated with subclinical LV dysfunction in African-Americans, even in the setting of preserved LV ejection fraction.
Keywords: BMI, LV function, heart failure, cardiovascular disease, Jackson Heart Study
INTRODUCTION
Obesity disproportionately affects African-American individuals with approximately 50% having a body mass index (BMI) greater than 30 kg/m2 (1). BMI is an established risk factor for cardiovascular disease, including heart failure (2). Obesity is associated with cardiac remodeling and hemodynamic changes collectively referred to as obesity cardiomyopathy (3-6). Although previously characterized in European Americans, whether higher BMI is associated with left ventricular systolic dysfunction in African-Americans is not well understood (7).
Advances in non-invasive cardiac imaging enable the quantification of myocardial mechanics, which can be used to characterize subtle changes in cardiac motion. Evaluation of LV function can be performed by measuring myocardial strain (i.e. deformation). Strain has been demonstrated to be a more sensitive method than LV ejection fraction (LVEF) for the detection of subclinical cardiac dysfunction (8). Evidence from small echocardiographic studies suggest that obesity may be associated with subclinical LV systolic dysfunction, as measured by strain imaging, even in the setting of preserved or increased LV ejection fraction (8-10). Tagged cardiac magnetic resonance imaging (MRI) is an alternative to echocardiography for the measurement of LV myocardial mechanics that may be particularly informative in understanding the relationship between BMI and cardiac structure function, as it is less susceptible to poor imaging windows in obese individuals compared with transthoracic echocardiography (11).
Despite the high prevalence of obesity in African-American individuals, the relationship between BMI and myocardial mechanics remains understudied in this population. The Jackson Heart Study (JHS) cohort of African-American adults who participated in the cardiac MRI exam (2008-2012) provides a unique opportunity to test the hypothesis that higher BMI is associated with subclinical LV systolic dysfunction, as measured by global circumferential strain (Ecc).
METHODS
Study Population
The JHS is a prospective observational cohort study designed to study the causes of cardiovascular disease in African-Americans. Study design and protocols have been previously described (12). Briefly, between 2000 to 2004, adult African-American men and women, aged 35-84 years (n=5,301), were recruited from metropolitan Jackson, Mississippi to participate in the baseline exam. The JHS cardiac magnetic resonance imaging (cMRI) component began in 2008 at the end of visit 2 and continued through visit 3 ending in December 2012. Exclusion for the cardiac MRI component included pregnancy, contraindication to MRI (implanted electrical devices, pacemaker, history of metal around orbit, etc), claustrophobia, or were unable to fit in the MRI machine. A total of 1,672 JHS participants completed the cardiac MRI. Participants in whom Eularian circumferential systolic strain (Ecc) was missing (n=20) were excluded, resulting in the final sample of 1,652 participants (n=253 from visit 2 and n=1399 from visit 3). There were no other exclusion criteria. The study protocol was approved by the Institutional Review Boards of Jackson State University, Tougaloo College, and the University of Mississippi Medical Center, and all study participants provided written informed consent.
Cardiac MRI
Cardiac MRI was performed with a large bore (70 cm), high-gradient, 1.5-T magnet (Siemens Espree with TIM Cardiac Package, Siemens Medical Solutions USA Inc., Malvern, PA) using a multi-channel matrix surface coil to obtain short- and long-axis electrocardiogram (ECG) gated steady-state free precession CINE images of the heart using a standardized protocol for measurement of cardiac structure and function developed in conjunction with the cMRI exam performed as part the NHLBI Multi-Ethnic Study of Atherosclerosis (MESA) exams 2 and 3 cMRI. Protocols were standardized for potential future comparison studies with JHS and MESA. Cardiac MRI data for the present analysis was not shared with MESA and the strain data from the JHS cohort has not been previously published. Sequences for functional assessment were obtained during a short breath-hold using white blood sequences, Fast imaging with Steady Procession (TrueFISP or TRUFI, Siemens sequence variant Tfi2d1_18) with the following parameters: FOV 400 mm, slice thickness 8 mm, matrix 109 × 192, TR 45.5 msec, TE 1.1 msec, flip angle 78-82 degrees. Additional single breath-hold, ECG gated, short axis sequences located at the cardiac base, mid and apex were obtained using a CINE radiofrequency grid tagging sequence with the following parameters: FOV 400 mm, slice thickness 8 mm, 192 × 256 matrix, TR 60 sec, TE 4 sec, flip angle 12 degrees (Siemens Sequence: Tl2d1r5). Radiofrequency pulses were used to “tag” the myocardium during cardiac MRI.
Global Circumferential Strain (Ecc), LV structure and function
Cardiac MRI scans were centrally analyzed at the Wake Forest University School of Medicine (Winston-Salem, NC). Cardiac Image Modeller software (Auckland UniServices Limited, Auckland, New Zealand) was used to measure LV mass, end diastolic volume (EDV) and end systolic volume (ESV), from which stroke volume (SV), LVEF, and LV remodeling index (LVRI = mass/EDV) were calculated.
Diagnasoft Harmonic Phase software (HARP, Research Triangle Park, Morrisville, NC) was used to quantify Ecc strain. Tagged tissues in the mid-myocardium were tracked through systole as an assessment of Ecc (11). HARP utilizes the k-space data and specifically the phase information to create a binary image that facilitates semi-automated detection of the tag lines throughout the cardiac cycle and has been previously used to determine subclinical LV dysfunction (13-15). Global peak Ecc was determined as the average of mid-wall strain in the base, mid, and apical LV regions from short-axis images.
Regional myocardial circumferential strain was defined by the Lagrangian formula: [e=(L−Lo)/Lo], where e is strain, Lo is the baseline length, and L is the instantaneous length at the time of measurement. Consequently, negative strain occurs if the measured segment length is shorter than its original length in the circumferential direction. Therefore, global circumferential systolic strain values closer to zero (less negative) are indicative of worse LV function.
Reproducibility of cardiac MRI measures using HARP was assessed on 96 scans blind duplicate exams that had their DICOM headers relabeled and were inserted into the workflow as newly received exams. Interclass correlation coefficients (ICC) for global Ecc analysis was 0.78 ICC’s for EDV, ESV, and LV mass, were 0.95, 0.88, and 0.96, respectively.
BMI and Covariates
BMI and covariates were ascertained from the closest visit preceding the date of acquisition of cardiac MRI data. BMI was defined as weight (kg) divided by height2 (m2). Fasting blood samples were collected according to standardized procedures and the assessment of glucose, lipids, insulin, creatinine, CRP, P-selectin, and E-selectin were processed at a central laboratory as previously described (12,16). Blood pressure was taken from the baseline exam as the average of two measures recorded at 5-minute intervals while seated.
Hypertension was defined by a self-reported diagnosis of hypertension, blood pressure > 140/90 mmHg, or treatment with antihypertensive medications. Diabetes mellitus was defined by fasting plasma glucose ≥126 mg/dL, HbA1c ≥ 6.5%, or treatment with insulin or oral hypoglycemic agent. Smoking status was determined by self-report and categorized as ever or never. Cardiovascular disease status was considered present if the participant reported a history of myocardial infarction, abnormal stress test, prior coronary artery bypass graft surgery, prior coronary angioplasty, or history of stroke at any visit. Estimated glomerular filtration rate (eGFR) at exam 3 (n=1,389) was calculated from serum creatinine using the CKD-EPI equation (17). Chronic kidney disease (CKD) was defined as an eGFR ≤ 60 ml/min/1.73m2. Plasma E-selectin and P-selectin were quantified by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota, USA). The interassay coefficients of variation for the E-selectin and P-selectin detection methods were 9.78% and 5.14%, respectively (18). CRP was determined using immunoturbidimetric CRP-Latex assay from Kamiya Biomedical Company following the manufacturer's high-sensitivity protocol. The inter-assay coefficients of variation on control samples repeated in each assay were 4.5% and 4.4% at CRP concentrations of 0.45 mg/L and 1.56 mg/L, respectively. The reliability coefficient for masked quality control replicates was 0.95 for the CRP assay (19).
Visceral adipose tissue (VAT) was measured at visit 2 (n = 263) from multi detector CT imaging (GE Heathcare Lightspeed 16 Pro, Milwaukee, Wisconsin) as previously described (20). Briefly, twenty-four continuous 2.5-mm thick image slices were acquired covering 60 mm above the level of S1. The volumetric quantification of VAT was performed using the volume Analysis software (Advantage Windows, GE Healthcare, Waukesha, WI, USA) and the sum of VAT pixels over 24 slices were calculated as the volumes of VAT.
Statistical Analysis
Descriptive statistics of demographic, clinical, and cardiac MRI variables were stratified by BMI according the World Health Organization defined categories as follows, lean (BMI < 25 kg/m2), overweight (25 to < 30 kg/m2), class I obese (30 to < 35 kg/m2), and class II or III obese (≥ 35 kg/m2). Results are presented as mean and standard deviation or percentages, as appropriate. One-way ANOVA was performed for continuous variables and chi squared test for categorical variables. Correlations between BMI and Ecc were examined using Spearman rank correlation coefficients.
Sequential multivariable-adjusted linear regression models were used to examine the association between BMI on a continuous scale and Ecc. Both BMI and Ecc were normally distributed and did not require transformation. Covariates were chosen a priori based on previous reports of factors associated with myocardial mechanics (6,14,21) and included age, gender, diabetes mellitus, smoking, hypertension, LDL-C, triglycerides, heart rate, eGFR, LVEF, and LV mass. Interaction terms were included in the multivariable-adjusted models to assess whether the association between BMI and Ecc differed in pre-specified subgroups of age, gender, BMI, hypertension, diabetes, CVD history, and chronic kidney disease. Multivariable-adjusted restricted cubic spline analysis with four knots was performed to graphically display and evaluate for non-linear associations between BMI on a continuous scale with Ecc or LVEF. The number of knots was selected based upon the model that produced the lowest Akaike Information Criterion (AIC).
In exploratory analyses, potential mechanisms for the association between BMI and Ecc were examined with additional models adjusted for 1) inflammatory markers (E selectin, P selectin, and CRP); 2) insulin resistance, measured by the homeostasis model of insulin resistance (HOMA-IR) among non diabetics (n=915); and 3) VAT (n=263) as a primary fat depot measurement.
A sensitivity analysis was performed to account for the heterogeneity in time difference between cardiac MRI and the clinical visit based on the following subgroups: <1 year (n=1076), <6 months (n=1011), and <1 month (n=597). All statistical analyses were conducted with STATA version 12.0. A two-tailed level of significance was set at P < 0.05.
RESULTS
Baseline characteristics and cardiac structure and function
Obesity was common, present in 55% of participants (Table 1). Age was similar across the spectrum of BMI. The proportion of females was higher, while the frequency of smoking was lower with higher BMI. Increased BMI was also associated with higher prevalence of diabetes and hypertension and with higher heart rate and blood pressure measurements. Prior history of cardiovascular disease and lipid levels were similar across the range of BMI. Unadjusted, left ventricular volume, mass, LVEF, and LVRI was higher with increased BMI (Table 2).
Table 1.
Clinical characteristics of Jackson Heart Study participants of the cardiac MRI exam.
| Variable |
BMI<25
(n=195) |
BMI 25-30
(n=551) |
BMI 30-35
(n=482) |
BMI >35
(n=424) |
P |
|---|---|---|---|---|---|
| Age, years | 59.7 ± 10.7 | 60.4 ± 10.4 | 59.3 ± 10.2 | 57.6 ± 10.1 | 0.71 |
| Females | 57 | 51 | 63 | 80 | <0.0001 |
| CVD | 6.7 | 7.1 | 9.1 | 8 | 0.59 |
| Ever Smoked | 36.4 | 28.3 | 27.6 | 24.3 | 0.02 |
| Hypertension | 59.4 | 72.6 | 75.2 | 81.5 | <0.0001 |
| Diabetes mellitus | 7.8 | 21.4 | 28.2 | 40.3 | <0.0001 |
| Anti-HTN med* | 52 | 65 | 68 | 77 | <0.0001 |
| Anti-diabetic med* | 7 | 17 | 22 | 29 | <0.0001 |
| Statin * | 23 | 27 | 27 | 24 | 0.52 |
| Heart rate, bpm | 71 ± 12 | 70 ± 11 | 70 ± 10 | 73 ± 13 | <0.0001 |
| Systolic BP, mm Hg | 127 ± 19 | 126 ± 16 | 126 ± 18 | 128 ± 20 | <0.0001 |
| Diastolic BP, mm Hg | 75 ± 10 | 75 ± 11 | 76 ± 11 | 80 ± 46 | <0.0001 |
| Fasting glucose*, mg/dL | 94 ± 21 | 103 ± 34 | 105 ± 33 | 110 ± 39 | <0.0001 |
| Total Chol.*, mg/dL | 202 ± 39 | 197 ± 40 | 199 ± 40 | 196 ± 42 | 0.37 |
| LDL*, mg/dL | 119 ± 36 | 120 ± 36 | 124 ± 36 | 121 ± 37 | 0.88 |
| Triglyceride*, mg/dL | 87 ± 58 | 95 ± 59 | 102 ± 62 | 100 ± 65 | 0.16 |
| eGFR*, ml/min/1.73m2 | 91 ± 20 | 88 ± 20 | 89 ± 21 | 91 ± 23 | 0.008 |
| Chronic Kidney Disease | 6.5 | 7.5 | 7.7 | 10.8 | 0.24 |
| HOMA-IR y, units | 1.70 ± 1.33 | 2.44 ± 1.56 | 3.35 ± 1.88 | 3.87 ± 2.12 | <0.0001 |
| CRP, mg/mL | 0.28 ± 0.52 | 0.33 ± 0.53 | 0.50 ± 0.60 | 0.93 ± 1.58 | <0.0001 |
| P-Selectin, ng/mL | 31.8 ± 11.0 | 32.1 ± 10.7 | 34.0 ± 11.7 | 34.3 ± 11.4 | 0.29 |
| E-selectin, ng/mL | 38.6 ± 17.0 | 41.5 ± 18.7 | 43.9 ± 18.3 | 46.9 ± 21.6 | <0.0001 |
| VAT (cm3) z | 547 ± 283 | 657 ± 250 | 896 ± 364 | 987 ± 409 | <0.0001 |
Data presented as mean ± SD or percentage.
>5% of participants missing.
only available for non diabetics.
only available for 246 participants from visit 2.
Table 2.
Cardiac Structure and Function in JHS Cardiac MRI Study participants.
| Variable |
BMI<25
(n=195) |
BMI 25-30
(n=551) |
BMI 30-35
(n=482) |
BMI >35
(n=424) |
P |
|---|---|---|---|---|---|
| LV mass, g | 113 ± 30 | 130 ± 37 | 138 ± 42 | 146 ± 42 | <0.0001 |
| EDV, ml | 115 ± 32 | 122 ± 35 | 126 ± 34 | 131 ± 36 | 0.23 |
| ESV, ml | 47 ± 18 | 50 ± 23 | 50 ± 20 | 51 ± 23 | <0.0001 |
| SV, ml | 68 ± 20 | 72 ± 20 | 75 ± 20 | 80 ± 22 | 0.061 |
| LVRI, g/ml | 1.02 ± 0.28 | 1.11 ± 0.30 | 1.13 ± 0.34 | 1.17 ± 0.37 | <0.0001 |
| LVEF, % | 59.4 ± 9.1 | 59.7 ± 9.6 | 60.6 ± 8.3 | 61.5 ± 9.3 | 0.01 |
| Ecc, % | −16.8 ± 2.5 | −16.4 ± 2.7 | −16.0 ± 2.6 | −15.5 ± 2.9 | 0.034 |
Data presented as Mean ± SD. ECC: systolic global circumferential strain, EDV: end diastolic volume, ESV: end systolic volume, LVEF: left ventricular ejection fraction, LVRI: left ventricular remodeling index (LV mass/EDV), SV: stroke volume
Association between BMI and global circumferential systolic strain: Multivariable analyses
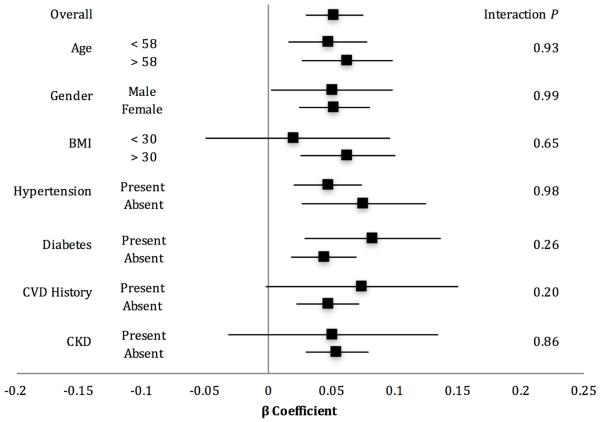
Higher BMI was associated with reduced LV systolic function as shown by less negative Ecc values, unadjusted (Table 2) and remained significant with adjustment for renal function, LVEF, and LV mass (Table 3). The association of higher BMI and LV systolic dysfunction was consistent between visits and across multiple clinically relevant subgroups, including age, sex, and traditional cardiovascular risk factors (Figure 1).
Table 3.
Association between BMI and global circumferential systolic strain among JHS cardiac MRI ancillary study participants.
| Model | Covariates | β (95% CI) | P |
|---|---|---|---|
| 1 | age, sex, DM, smoke, HTN*, TG, HR, CVD | 0.089 (0.066,0.112) | <0.0001 |
| 2 | Model 1 + eGFR | 0.072 (0.050,0.095) | <0.0001 |
| 3 | Model 2 + LVEF | 0.080 (0.059,0.102) | <0.0001 |
| 4 | Model 2 + LVEF and LV mass | 0.052 (0.028,0.075) | <0.0001 |
| 5 | Model 3 + LVRI | 0.052 (0.030,0.074) | <0.0001 |
Data presented with BMI β coefficient. Global circumferential systolic strain (Ecc) is measured on a negative scale, thus a positive β coefficient reflects worse strain.
HTN used instead of systolic blood pressure and BP medication status due to >5% missing data for those variables. Models were created using these parameters with no change in the association. CVD: cardiovascular disease history, DM: diabetes, EF: ejection fraction, HR: heart rate, HTN: hypertension, LVRI: LV remodeling index, smoke: ever smoked, TG: triglycerides.
Figure 1. Forest plot of the associations between BMI and global circumferential systolic strain, overall and by subgroups.
Forest plot of BMI beta coefficients by subgroups adjusting for covariates in regression model 3. 95% CI shown as solid horizontal line.
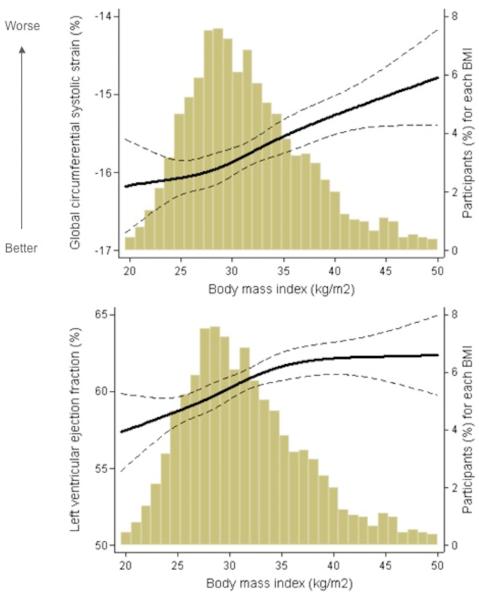
The relationships between BMI (range 15-69 kg/m2) and Ecc or LVEF were examined in multivariable-adjusted restricted cubic spline analysis (Figure 2). Higher BMI was related to higher LVEF (β=0.19; 95% CI: 0.094, 0.29; P < 0.0001). In contrast, across the range of BMI, greater BMI was associated with worsening of LV systolic function (Ecc), even in the setting of preserved and increasing LVEF.
Figure 2. Adjusted Regression Analysis of BMI with Ecc and LV Ejection Fraction.
(a) Adjusted cubic spline regression plot of global circumferential strain and BMI overlaid on a histogram illustrating the distribution of BMI among JHS participants. (b) Adjusted cubic spline regression plot of LVEF and BMI. Solid line depicts predicted values and dashed lines represent 95% CI.
Potential Mechanisms for Subclinical LV Dysfunction
In exploratory analyses, multivariable-adjusted linear regression models including available inflammatory biomarkers, CT measured visceral adipose tissue (VAT), and HOMA-IR did not significantly attenuate the association of BMI and subclinical LV dysfunction (Table 4).
Table 4.
Subclinical left ventricular dysfunction and BMI with further adjustment for inflammatory biomarkers, insulin resistance, and visceral adiposity.
| Model | Covariates | β for BMI (95% CI) | P |
|---|---|---|---|
| Inflammation | |||
| 1 | CVD risk factors + E selectin | 0.052 (0.028, 0.075) | <0.0001 |
| 2 | CVD risk factors + P selectin | 0.053 (0.030, 0.077) | <0.0001 |
| 3 | CVD risk factors + CRP | 0.052 (0.028, 0.076) | <0.0001 |
| Insulin Resistance | |||
| 4 | CVD risk factors + HOMA-IR | 0.035 (0.007, 0.063) | 0.013 |
| Adiposity Measure | |||
| 5 | CVD risk factors + VAT | 0.12 (0.046, 0.20) | 0.002 |
Data presented with BMI β coefficient. CRP: C-reactive protein, CVD risk factors: age, sex, diabetes, hypertension, ever smoked, triglycerides, heart rate, cardiovascular disease history, estimated glomerular filtration, LV mass, and ejection fraction, HOMA-IR: Homeostasis model of insulin resistance, VAT: Visceral Adipose Tissue.
DISCUSSION
In this large, community-based cohort of middle-aged African-American individuals, we found that higher BMI, across a range of BMI from 15-69 kg/m2, is significantly associated with subclinical LV systolic dysfunction as measured by cardiac MRI mid-wall circumferential strain. The relationship between BMI and LV dysfunction was independent of prevalent cardiovascular disease, traditional cardiometabolic risk factors, and LVEF, and was consistent in subgroup analyses. The significant association between BMI and Ecc persisted even after adjustment for inflammatory markers, insulin resistance, and abdominal visceral fat.
Several studies have reported that obesity is independently associated with alterations in cardiac structure and mechanics; however, they were conducted in predominantly white populations using echocardiography and did not include individuals with BMI >40 kg/m2 (8-10). Transthoracic echocardiography can be particularly challenging in obese patients due to acoustic window constraints from excess chest wall fat. Cardiac MRI, like echocardiography, is a non-invasive procedure, but it overcomes several of the limitations of echocardiography related to obesity (22,23). Our findings of impaired LV systolic function with higher BMI are consistent with several previous studies examining the relationship of obesity subgroups (classes I, II, and III) and LV strain patterns (7,10,24). However, there have been conflicting reports of alterations in myocardial mechanics for individuals with milder degrees of obesity (9,25-27). Our study suggests that the association of BMI and subclinical LV dysfunction is near linear across the range of BMI 27-50 kg/m2 in African-Americans. Possible explanations for the differences between our study and prior studies may be related to less sensitive techniques used to detect preclinical measures of LV dysfunction, lack of power in past study designs, the categorization of obesity subgroups as opposed to analyzing BMI on a continuous spectrum, and predominantly Caucasian populations.
Our findings further suggest that the association between BMI and Ecc was not explained by confounding from conditions predisposing to myocardial impairment. The association of higher BMI with LV dysfunction was consistent across multiple clinically relevant subgroups including age, sex, hypertension, diabetes, cardiovascular disease, and chronic kidney disease. Previous reports showed that comorbid conditions with obesity including hypertension and diabetes in asymptomatic patients are characterized by improved circumferential strain, which has been proposed as a compensatory mechanism to preserve LVEF (21,28-30). However, our findings suggest that higher BMI is associated with impaired circumferential strain independent of these comorbidities. This discrepancy suggests that obesity may contribute to subclinical LV dysfunction through a variety of mechanisms not limited to changes in loading conditions, insulin resistance, and coronary artery disease.
Although LVEF is the most commonly used measure of systolic function in clinical practice, it is highly load dependent and insensitive to subtle changes in cardiac function (31,32). Across the entire clinical range of BMI measured in our study population, LVEF was largely within the normal range (55-65%) and positively related to BMI after multivariable adjustment. As demonstrated in figure 2, above a BMI of 35 kg/m2, the relation between BMI and LVEF is effectively flat and in the normal range. In contrast, Ecc worsens in a near linear relationship across the entire spectrum of BMI despite a normal range of LVEF, supporting the concept that higher BMI is associated with altered myocardial mechanics.
Proposed mechanisms by which higher BMI may associate with adverse cardiac remodeling and dysfunction include inflammation, insulin resistance, and myocardial metabolic changes. Systemic release of inflammatory mediators from excess adipose tissue results in myocardial injury and subsequent cardiac structural alterations (2,33-35). 31P magnetic resonance spectroscopy has shown a myocardial energetic deficit exists in obesity likely resulting in impaired systolic function (36). Some studies suggest insulin resistance leads to these alterations in myocardial substrate metabolism, which contributes to the LV dysfunction associated with obesity (24,37,38). These metabolic derangements are characterized by increased myocardial fatty acid metabolism resulting in myocardial injury via lipotoxicity and concurrently decreased glucose metabolism (39-41). In addition, BMI may be a surrogate for the distribution of body fat and muscle, with some studies implicating visceral adiposity as a greater cardiovascular risk factor than BMI (42). Visceral adiposity may better represent both myocardial triglyceride content and LV mass, which was shown to partially attenuate the association of BMI and ECC in our models. We examined whether circulating markers of inflammation, insulin resistance, or visceral adipose tissue mitigated the association between BMI and worsened Ecc. While insulin resistance and visceral adiposity attenuated the magnitude of the relationship, these factors only partially accounted for the relationship between BMI and Ecc, suggesting alternate unmeasured factors may also be involved.
We examined a large, well-phenotyped adult African-American population age 35 to 84 years and spanning a wide BMI 15-69 kg/m2 to quantify the relationship between BMI and subclinical LV systolic dysfunction using tagged cardiac MRI. To our knowledge, our study is the first to examine the association of BMI on a continuous scale and subclinical LV dysfunction using cardiac MRI in African-Americans. Our study is unique in that we were able use the large bore Siemens Espree MRI machine so as to include JHS participants with BMI>50 kg/m2, which was not possible in previous studies, such as the Multi-Ethnic Study of Atherosclerosis. Despite the strengths of our study, limitations should be noted. We could not determine causal relationships between BMI and left ventricular dysfunction given the cross-sectional study design. Measurements of longitudinal systolic strain, which may be a more sensitive measure than Ecc for the detection of changes in myocardial deformation, were not available in the JHS population, but are a future direction. Cardiac MRI studies were not obtained on the same day as ascertainment of BMI and clinical covariates. Differing myocardial preload, afterload, and contractile state at the time of the MRI exam could affect Ecc and confound the analysis. We accounted for preload and afterload with heart rate and LVRI, which were taken at the time of MRI. Blood pressure was not measured concurrently with the cardiac MRI exams; however, controlling for another measure of afterload, LVRI, the relationship between BMI and Ecc was still significant. In addition, adjusting for the time difference between imaging and covariate measurements did not change the inference. BMI may not account for distributions of body fat or other proxy measures of obesity, such as waist circumference or waist to hip ratio, which may have provided different information. However, waist and hip circumference have greater measurement error and are less commonly used in routine clinical practice compared with BMI, therefore were not the focus of this analysis. Despite accounting for inflammation, insulin resistance, and visceral adipose tissue, the precise mechanisms underlying the association between BMI and subclinical left ventricular dysfunction remain unknown.
CONCLUSION
In a community based cohort of African-American individuals, higher BMI was significantly associated with subclinical LV dysfunction independent of traditional cardiometabolic risk factors, renal function, cardiac structural characteristics, and LVEF. BMI may not only reflect obesity, but also pathologic processes that result in impaired myocardial mechanics. Better understanding of the pathophysiology of how higher BMI relates to cardiomyopathy should inform strategies to prevent and potentially reverse detrimental subclinical LV alterations.
CLINICAL PERSPECTIVES.
Obesity disproportionately affects the African-American population and is associated with adverse cardiac remodeling and hemodynamic changes. BMI is an established risk factor for cardiovascular disease and heart failure, but the relationship between BMI and myocardial mechanics remains understudied in this population. We found that higher BMI is significantly associated with subclinical LV systolic dysfunction in African-American individuals.
TRANSLATIONAL OUTLOOK.
Future studies to clarify the pathophysiology of how higher BMI relates to cardiomyopathy are warranted to inform strategies to prevent and potentially reverse detrimental subclinical LV alterations.
ACKNOWLEDGEMENTS
Disclosures:
The Jackson Heart Study is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. The authors thank the participants and data collection staff of the Jackson Heart Study. Research reported in this paper was supported by NIH grants K12 HL109019, K23 HL128928-01A1, and R01-HL-102780.
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Abbreviations
- JHS
Jackson Heart Study
- MRI
magnetic resonance imaging
- BMI
body mass index
- ECC
global circumferential strain
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- LVRI
left ventricular remodeling index
- HOMA-IR
homeostasis model of insulin resistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2016 Update: A Report From the American Heart Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Ndumele CE, Coresh J, Lazo M, et al. Obesity, subclinical myocardial injury, and incident heart failure. JACC Heart failure. 2014;2:600–7. doi: 10.1016/j.jchf.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russo C, Jin Z, Homma S, et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. Journal of the American College of Cardiology. 2011;57:1368–74. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakhani M, Fein S. Effects of obesity and subsequent weight reduction on left ventricular function. Cardiology in review. 2011;19:1–4. doi: 10.1097/CRD.0b013e3181f877d2. [DOI] [PubMed] [Google Scholar]
- 5.Ballo P, Motto A, Mondillo S, Faraguti SA. Impact of obesity on left ventricular mass and function in subjects with chronic volume overload. Obesity. 2007;15:2019–26. doi: 10.1038/oby.2007.241. [DOI] [PubMed] [Google Scholar]
- 6.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. The American journal of the medical sciences. 2001;321:225–36. doi: 10.1097/00000441-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–7. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 8.Buckberg G, Hoffman JI, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–87. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 9.Pascual M, Pascual DA, Soria F, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–6. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scaglione R, Dichiara MA, Indovina A, et al. Left ventricular diastolic and systolic function in normotensive obese subjects: influence of degree and duration of obesity. European heart journal. 1992;13:738–42. doi: 10.1093/oxfordjournals.eurheartj.a060249. [DOI] [PubMed] [Google Scholar]
- 11.Jeung MY, Germain P, Croisille P, El ghannudi S, Roy C, Gangi A. Myocardial tagging with MR imaging: overview of normal and pathologic findings. Radiographics : a review publication of the Radiological Society of North America, Inc. 2012;32:1381–98. doi: 10.1148/rg.325115098. [DOI] [PubMed] [Google Scholar]
- 12.Taylor HA, Jr., Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethnicity & disease. 2005;15 S6-4-17. [PubMed] [Google Scholar]
- 13.Fernandes VR, Polak JF, Edvardsen T, et al. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA) Journal of the American College of Cardiology. 2006;47:2420–8. doi: 10.1016/j.jacc.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 14.Kishi S, Armstrong AC, Gidding SS, et al. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the CARDIA study (Coronary Artery Risk Development in Young Adults) JACC Heart failure. 2014;2:500–8. doi: 10.1016/j.jchf.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edvardsen T, Detrano R, Rosen BD, et al. Coronary artery atherosclerosis is related to reduced regional left ventricular function in individuals without history of clinical cardiovascular disease: the Multiethnic Study of Atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:206–11. doi: 10.1161/01.ATV.0000194077.23234.ae. [DOI] [PubMed] [Google Scholar]
- 16.Fuqua SR, Wyatt SB, Andrew ME, et al. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethnicity & disease. 2005;15 S6-18-29. [PubMed] [Google Scholar]
- 17.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. Jama. 2012;307:1941–51. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penman A, Hoadley S, Wilson JG, Taylor HA, Chen CJ, Sobrin L. P-selectin Plasma Levels and Genetic Variant Associated With Diabetic Retinopathy in African Americans. American journal of ophthalmology. 2015;159:1152–1160. doi: 10.1016/j.ajo.2015.03.008. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox ER, Benjamin EJ, Sarpong DF, et al. The relation of C--reactive protein to chronic kidney disease in African Americans: the Jackson Heart Study. BMC nephrology. 2010;11:1. doi: 10.1186/1471-2369-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. The Journal of clinical endocrinology and metabolism. 2010;95:5419–26. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clinical science. 2004;106:53–60. doi: 10.1042/CS20030153. [DOI] [PubMed] [Google Scholar]
- 22.Jiang K, Yu X. Quantification of regional myocardial wall motion by cardiovascular magnetic resonance. Quantitative imaging in medicine and surgery. 2014;4:345–57. doi: 10.3978/j.issn.2223-4292.2014.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoneyama K, Gjesdal O, Choi EY, et al. Age, sex, and hypertension-related remodeling influences left ventricular torsion assessed by tagged cardiac magnetic resonance in asymptomatic individuals: the multi-ethnic study of atherosclerosis. Circulation. 2012;126:2481–90. doi: 10.1161/CIRCULATIONAHA.112.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson LR, Herrero P, Schechtman KB, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–6. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 25.Willens HJ, Chakko SC, Lowery MH, et al. Tissue Doppler imaging of the right and left ventricle in severe obesity (body mass index >35 kg/m2) The American journal of cardiology. 2004;94:1087–90. doi: 10.1016/j.amjcard.2004.06.076. [DOI] [PubMed] [Google Scholar]
- 26.Di Bello V, Santini F, Di Cori A, et al. Obesity cardiomyopathy: is it a reality? An ultrasonic tissue characterization study. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2006;19:1063–71. doi: 10.1016/j.echo.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 27.Iacobellis G, Ribaudo MC, Leto G, et al. Influence of excess fat on cardiac morphology and function: study in uncomplicated obesity. Obesity research. 2002;10:767–73. doi: 10.1038/oby.2002.104. [DOI] [PubMed] [Google Scholar]
- 28.Imbalzano E, Zito C, Carerj S, et al. Left ventricular function in hypertension: new insight by speckle tracking echocardiography. Echocardiography. 2011;28:649–57. doi: 10.1111/j.1540-8175.2011.01410.x. [DOI] [PubMed] [Google Scholar]
- 29.Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two-dimensional strain imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2008;21:1138–44. doi: 10.1016/j.echo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. European heart journal. 2012;33:1716–7. doi: 10.1093/eurheartj/ehs124. [DOI] [PubMed] [Google Scholar]
- 31.Aurigemma GP, Zile MR, Gaasch WH. Contractile behavior of the left ventricle in diastolic heart failure: with emphasis on regional systolic function. Circulation. 2006;113:296–304. doi: 10.1161/CIRCULATIONAHA.104.481465. [DOI] [PubMed] [Google Scholar]
- 32.Carabello BA. Evolution of the study of left ventricular function: everything old is new again. Circulation. 2002;105:2701–3. doi: 10.1161/01.cir.0000021240.86593.9d. [DOI] [PubMed] [Google Scholar]
- 33.Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. American journal of physiology Endocrinology and metabolism. 2012;303:E937–49. doi: 10.1152/ajpendo.00061.2012. [DOI] [PubMed] [Google Scholar]
- 34.Hatem SN, Sanders P. Epicardial adipose tissue and atrial fibrillation. Cardiovascular research. 2014;102:205–13. doi: 10.1093/cvr/cvu045. [DOI] [PubMed] [Google Scholar]
- 35.Wang HT, Liu CF, Tsai TH, et al. Effect of obesity reduction on preservation of heart function and attenuation of left ventricular remodeling, oxidative stress and inflammation in obese mice. Journal of translational medicine. 2012;10:145. doi: 10.1186/1479-5876-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rider OJ, Francis JM, Ali MK, et al. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation. 2012;125:1511–9. doi: 10.1161/CIRCULATIONAHA.111.069518. [DOI] [PubMed] [Google Scholar]
- 37.Park SY, Cho YR, Kim HJ, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005;54:3530–40. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira-Junior SA, Martinez PF, Guizoni DM, et al. AT1 receptor blockade attenuates insulin resistance and myocardial remodeling in rats with diet-induced obesity. PloS one. 2014;9:e86447. doi: 10.1371/journal.pone.0086447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carley AN, Severson DL. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochimica et biophysica acta. 2005;1734:112–26. doi: 10.1016/j.bbalip.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 40.Masoud WG, Ussher JR, Wang W, et al. Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Cardiovascular research. 2014;101:30–8. doi: 10.1093/cvr/cvt216. [DOI] [PubMed] [Google Scholar]
- 41.Kim G, Jo K, Kim KJ, et al. Visceral adiposity is associated with altered myocardial glucose uptake measured by (18)FDG-PET in 346 subjects with normal glucose tolerance, prediabetes, and type 2 diabetes. Cardiovascular diabetology. 2015;14:148. doi: 10.1186/s12933-015-0310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith SR, Lovejoy JC, Greenway F, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism: clinical and experimental. 2001;50:425–35. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]