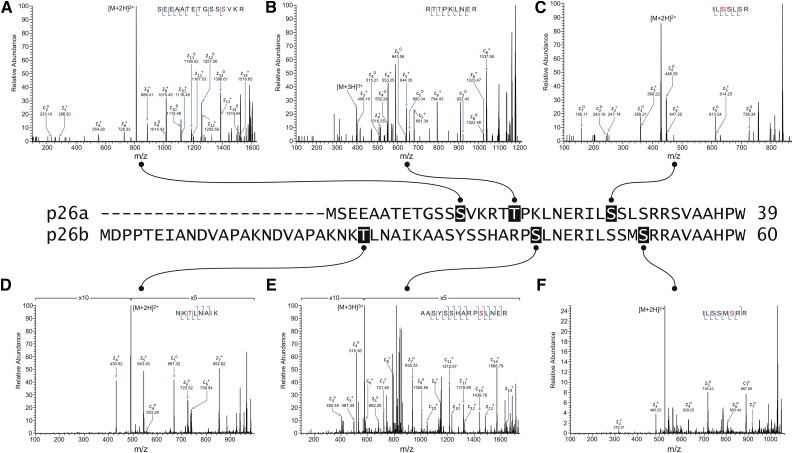
Figure 1.
Phosphorylation sites identified by LC-MS/MS in recombinant p26 phosphorylated in vitro by endogenous pollen kinases. The sequence alignment of the N-terminal regions of the p26 sPPase proteins is annotated to indicate amino acid residues identified as phosphorylated by endogenous pollen kinases by LC-MS/MS. A to F show ETD mass spectra detected from p26a (A–C) and p26b (D–F) after phosphorylation using pollen extracts. A, Ser-13 phosphorylation recorded on +2 ions at mass-to-charge ratio (m/z) 809.85. B, Thr-18 phosphorylation recorded on +3 ions at m/z 399.19. C, Ser-27 phosphorylation detected on +2 ions at m/z 428.22. D, Thr-25 phosphorylation detected on +2 ions at m/z 491.25. E, Ser-41 phosphorylation detected on +3 ions at m/z 575.92. F, Ser-51 phosphorylation detected on +2 ions at m/z 523.24. S/T, Phosphorylated residues.