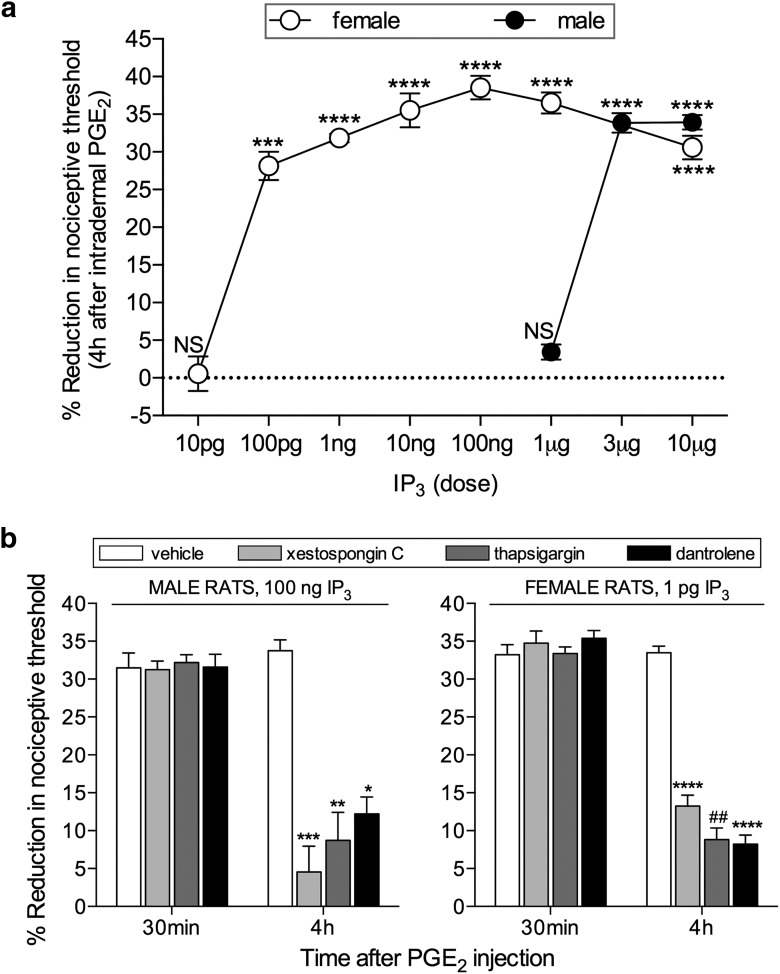
Figure 2.
Induction of hyperalgesic priming by IP3 in male and female rats. a, Different doses of IP3 were injected on the dorsum of the hindpaw in different groups of female (open circles represent 10 pg; 100 pg; 1 ng; 10 ng; 100 ng; 1 and 10 μg) and male (filled circles represent 1 μg; 3 μg; 10 μg) rats. No change in the mechanical nociceptive threshold was observed after the injection of IP3 (data not shown). PGE2 (100 ng) was injected at the same site, 1 week later, and the mechanical hyperalgesia evaluated by the Randall-Sellitto paw-withdrawal test. This figure shows the mechanical hyperalgesia at the fourth hour after the injection of PGE2; the presence of hyperalgesia at that time point was used to confirm the induction of priming by the previous injection of IP3. In the groups of female rats that had received a dose of 100 pg and higher, and in the group of male rats previously treated with 3 or 10 μg, but not with 1 μg, the hyperalgesia induced by PGE2 was still present at the fourth hour (female rats: 10 pg, t(5) = 1.627, p = 0.1647 (not significant, NS); 100 pg, t(5) = 8.788, p = 0.0003 (***); 1 ng, t(5) = 27.81; 10 ng, t(5) = 12.59; 100 pg, t(5) = 21.24; 1 μg, t(5) = 15.53; 10 μg, t(5) = 13.94, all p < 0.0001 (****); male rats: 1 μg, t(5) = 0.4385, p = 0.6793 (NS); 3 μg, t(5) = 16.54; 10 μg, t(5) = 23.27, both p < 0.0001 (****), when the mechanical nociceptive thresholds before and 4 h after the injection of PGE2, for each group, are compared, paired Student's t test). These results support the suggestion that nociceptors in females are significantly more sensitive to induction of priming by IP3 because a dose much lower was required. b, Groups of male (left) and female (right) rats received an injection of vehicle (white bars), the IP3 receptor inhibitor xestospongin C (0.2 μg, light gray bars), the SERCA inhibitor thapsigargin (1 μg, dark gray bars), or the ryanodine receptor inhibitor dantrolene (1 μg, black bars) on the dorsum of the hindpaw. Ten minutes later, the smallest doses of IP3 that induced priming (a; 3 μg in male; 100 pg in female) were injected at the same site. No significant change in mechanical nociceptive threshold was observed after injection of IP3 (data not shown). One week later, the presence of priming was determined by the evaluation of the prolonged mechanical hyperalgesia induced by intradermal injection of PGE2 (100 ng), at the same site as IP3. Two-way repeated-measures ANOVA, followed by Bonferroni post hoc test, showed that, while the hyperalgesia induced by PGE2 in control groups (vehicle-treated) was still present 4 h after injection, and not different from the 30 min time point, in the groups pretreated with xestospongin C, thapsigargin, or dantrolene, its magnitude was significantly smaller at the 4 h time point (males, xestospongin C group: F(1,5) = 81.30, ***p = 0.0003; thapsigargin group: F(1,5) = 31.11, **p = 0.0026; dantrolene group: F(1,5) = 44.44, *p = 0.0011; females: xestospongin C group: F(1,5) = 230.5, ****p < 0.0001; thapsigargin group: F(1,5) = 58.63, ##p = 0.0006; dantrolene group: F(1,5) = 16.48, ****p < 0.0001, when the hyperalgesia in the vehicle- and inhibitor-treated groups is compared at the fourth hour), indicating that the induction of priming by IP3 is dependent on the activation of IP3 and ryanodine receptors (N = 6 paws all groups).