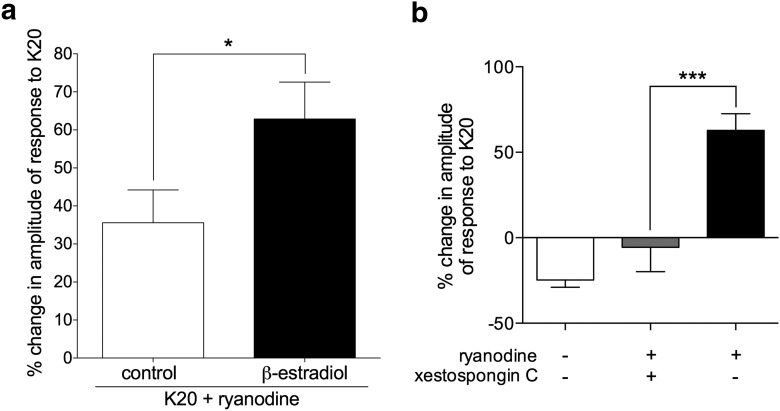
Figure 6.
Potentiation of depolarization-induced calcium transients by ryanodine is dependent on IP3 receptors and enhanced in the presence of β-estradiol. a, Pooled relative changes in the amplitude of the second response to K20 after exposure to ryanodine (2 nm) in IB4+ small DRG neurons, incubated without (control, white bar, N = 36) or in the presence of β-estradiol (100 nm, black bar, N = 28), reveal significant potentiation in both groups (positive values significantly >0: control group, t(35) = 4.1, p = 0.0002; β-estradiol group, t(27) = 6.5, p < 0.0001). Importantly, potentiation was significantly higher in the group incubated with β-estradiol (t(58) = 2.1, *p = 0.04) when both groups are compared (two-tailed unpaired Student's t test with Welch's correction). b, Pooled relative changes in the amplitude of the second response to K20 after exposure to ryanodine (2 nm) in IB4+ small DRG neurons, incubated with β-estradiol (100 nm), show lack of potentiation in neurons preincubated with the selective IP3 receptor antagonist xestospongin C (1 μm, gray bar; N = 8). Black bar is the same as the “β-estradiol” bar in a. White bar (no ryanodine) is the same as “β-estradiol alone” from Figure 4b, shown here for comparison. Xestospongin C significantly inhibited the ryanodine-induced potentiation of calcium transients compared with the control group (black bar) (F(2,61) = 35, ***p = 0.0004; one-way ANOVA followed by Tukey's multiple comparison test).